当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A General Approach to Deboronative Radical Chain Reactions with Pinacol Alkylboronic Esters.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-05 , DOI: 10.1002/anie.202004012 Emy André-Joyaux 1 , Andrey Kuzovlev 1 , Nicholas D C Tappin 1 , Philippe Renaud 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-05 , DOI: 10.1002/anie.202004012 Emy André-Joyaux 1 , Andrey Kuzovlev 1 , Nicholas D C Tappin 1 , Philippe Renaud 1
Affiliation
|
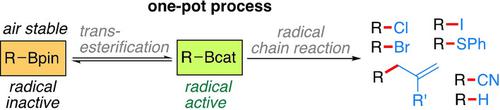
The generation of carbon‐centered radicals from air‐sensitive organoboron compounds through nucleohomolytic substitution at boron is a general method to generate non‐functionalized and functionalized radicals. Due to their reduced Lewis acidity, alkylboronic pinacol esters are not suitable substrates. We report their in situ conversion into alkylboronic catechol esters by boron‐transesterification with a substoichiometric amount of catechol methyl borate combined with an array of radical chain processes. This simple one‐pot radical‐chain deboronative method enables the conversion of pinacol boronic esters into iodides, bromides, chlorides, and thioethers. The process is also suitable the formation of nitriles and allylated compounds through C−C bond formation using sulfonyl radical traps. The power of combining radical and classical boron chemistry is illustrated with a modular 5‐membered ring formation using a combination of three‐component coupling and protodeboronative cyclization.
中文翻译:

用品那科烷基硼酸酯进行去硼自由基链反应的一般方法。
由空气敏感的有机硼化合物通过硼的核均热取代生成以碳为中心的自由基是生成未官能化和官能化自由基的通用方法。由于降低的路易斯酸度,烷基硼酸频哪醇酯不是合适的底物。我们报道了它们通过硼亚基酯交换与亚化学计量的邻苯二酚甲基硼酸酯结合一系列自由基链过程而原位转化为烷基硼邻苯二酚酯。这种简单的单锅自由基链脱硼方法可将频哪醇硼酸酯转化为碘化物,溴化物,氯化物和硫醚。该方法也适用于使用磺酰基自由基捕获剂通过C-C键形成腈和烯丙基化的化合物。
更新日期:2020-05-05
中文翻译:

用品那科烷基硼酸酯进行去硼自由基链反应的一般方法。
由空气敏感的有机硼化合物通过硼的核均热取代生成以碳为中心的自由基是生成未官能化和官能化自由基的通用方法。由于降低的路易斯酸度,烷基硼酸频哪醇酯不是合适的底物。我们报道了它们通过硼亚基酯交换与亚化学计量的邻苯二酚甲基硼酸酯结合一系列自由基链过程而原位转化为烷基硼邻苯二酚酯。这种简单的单锅自由基链脱硼方法可将频哪醇硼酸酯转化为碘化物,溴化物,氯化物和硫醚。该方法也适用于使用磺酰基自由基捕获剂通过C-C键形成腈和烯丙基化的化合物。















































 京公网安备 11010802027423号
京公网安备 11010802027423号