Dyes and Pigments ( IF 4.1 ) Pub Date : 2020-05-05 , DOI: 10.1016/j.dyepig.2020.108504 Guangxian Su , Kaiqi Zhang , Feng Sha , Zhangfa Tong , Jia Ni , Chengjie Li , Qizhao Li , Yu Du , Xiaoming Cao , Xin-Yan Wu , Yongshu Xie
|
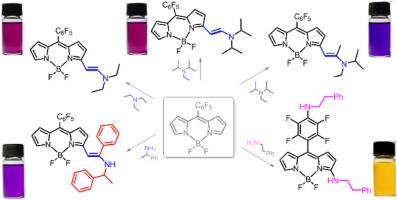
Direct functionalization of meso-C6F5 BODIPY with various enamines and amines at the pyrrolic α-position has been achieved by reacting the BODIPY with ethylamines in acetonitrile using BPO (benzoyl peroxide) as an additive under aerobic atmosphere at room temperature. The reaction modes of the products can be effectively modulated by the substituents on the ethylamines, affording three different types of products with enamine and amine moieties regioselectively attached at the pyrrolic α position and the para-position of the meso-C6F5 group. The spectroscopic properties are effectively modulated by the ICT effect from the electron-donating enamino or amino groups to the electron-withdrawing BODIPY core and the pentafluorophenyl moiety, with the absorption and emission maxima varying in the ranges of 497–602 and 535–650 nm, respectively. These results compose an efficient method for synthesizing enamine and amine functionalized BODIPYs.
中文翻译:

通过与乙胺的一步反应将BODIPY与烯胺和胺官能化
通过在室温下好氧气氛下使用BPO(过氧化苯甲酰)作为添加剂,使BODIPY与乙胺在乙腈中反应,已经实现了在各种不同的烯胺和吡咯α位上的内消旋-C 6 F 5 BODIPY的直接官能化。产物的反应模式可以通过乙胺上的取代基进行有效调节,从而提供三种不同类型的产物,其中烯胺和胺部分区域选择性地连接在内消旋-C 6 F 5的吡咯α位和对位组。从给电子的烯氨基或氨基到吸电子的BODIPY核和五氟苯基部分的ICT效应有效地调节了光谱性质,吸收和发射最大值在497-602和535-650 nm范围内变化, 分别。这些结果构成了合成烯胺和胺官能化的BODIPY的有效方法。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号