Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-05-05 , DOI: 10.1016/j.apcatb.2020.119088 Wei Hong , Mingpan Shao , Tianle Zhu , Haining Wang , Ye Sun , Fangxia Shen , Xiang Li
|
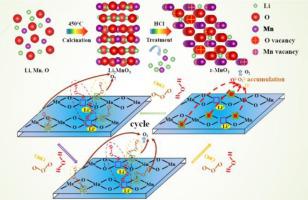
ε-MnO2 catalysts with Mn vacancies and appropriate Li+ were designed with the help of density functional theory calculations (DFT) and prepared by a novel selective dissolution strategy. Their physiochemical properties were characterized and catalytic activity for ozone decomposition evaluated. DFT calculations showed that Mn vacancies and appropriate amounts of Li+ in ε-MnO2 facilitated the formation of oxygen vacancies, decreased adsorption ability for H2O and O2, and maintained high adsorption ability for O3 on these oxygen vacancies. Characterization results showed that preparing ε-MnO2 by selective dissolution of Mn-Li precursors with 0.5 M HCl produced more Mn vacancies, and, thus, weaker crystallinity, larger specific surface area, superior reducibility, better oxygen storage capacity, and higher oxygen vacancies. This catalyst exhibited excellent activity and stability for ozone decomposition. Furthermore, a possible mechanism for ozone decomposition by the synergy of Li+, Mn vacancies, and oxygen vacancies in ε-MnO2 was proposed.
中文翻译:

为了通过制造锰空缺在ε-MnO的促进臭氧的催化分解2催化剂经由锰锂的前体的选择性溶解
ε-的MnO 2层的催化剂与锰空位和适当栗+在设计时密度泛函理论计算(DFT)的帮助和通过新颖的选择性溶解的策略制备。表征了它们的理化性质并评估了臭氧分解的催化活性。DFT计算表明,锰空位和适量的Li +在ε-的MnO 2促进氧空位的形成,降低吸附能力用于h 2 O和O 2,并保持高的吸附能力对于O- 3上这些氧空位。表征结果表明,制备ε-的MnO 2通过用0.5 M HCl选择性溶解Mn-Li前体产生更多的Mn空位,因此,较弱的结晶度,更大的比表面积,优异的还原性,更好的储氧能力和更高的氧空位。该催化剂显示出优异的臭氧分解活性和稳定性。此外,对于由Li的协同作用的臭氧分解的可能机制+,锰空位,和氧空位在ε-的MnO 2中提出的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号