Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2020-05-05 , DOI: 10.1016/j.apcata.2020.117599 Hai-Min Shen , Meng-Yun Hu , Lei Liu , Bei Qi , Hong-Liang Ye , Yuan-Bin She
|
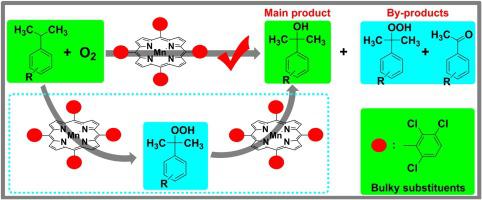
The direct and efficient oxidation of tertiary benzylic CH bonds to alcohols with O2 was accomplished in the presence of metalloporphyrins as catalysts under solvent-free and additive-free conditions. Based on effective inhibition on the unselective autoxidation and deep oxidation, systematical investigation on the effects of porphyrin ligands and metal centers, and apparent kinetics study, the oxidation system employing porphyrin manganese(II) (T(2,3,6-triCl)PPMn) with bulkier substituents as catalyst, was regarded as the most promising and efficient one. For the typical substrate, the conversion of cumene could reach up to 57.6% with the selectivity of 70.5% toward alcohol, both of them being higher than the current documents under similar conditions. The superiority of T(2,3,6-triCl)PPMn was mainly attributed to its bulkier substituent groups preventing metalloporphyrins from oxidative degradation, its planar structure favoring the interaction between central metal with reactants, and the high efficiency of Mn(II) in the catalytic transformation of hydroperoxides to alcohols.
中文翻译:

金属卟啉在温和无溶剂条件下高效选择性地氧化叔苄基C
叔苄基C H键直接有效地氧化为具有O 2的醇在无溶剂和无添加剂的条件下,在金属卟啉作为催化剂的存在下完成。基于对非选择性自氧化和深度氧化的有效抑制作用,对卟啉配体和金属中心的影响的系统研究以及表观动力学研究,使用卟啉锰(II)(T(2,3,6-triCl)PPMn的氧化系统)以更大的取代基作为催化剂,被认为是最有前途和最有效的方法。对于典型的底物,枯烯的转化率可高达57.6%,对醇的选择性为70.5%,在相似的条件下,两者均高于当前文献。T(2,3,6-triCl)PPMn的优越性主要归因于其较大的取代基可防止金属卟啉氧化降解,































 京公网安备 11010802027423号
京公网安备 11010802027423号