当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Optimization, and Study of Small Molecules That Target Tau Pre-mRNA and Affect Splicing
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-05-04 , DOI: 10.1021/jacs.0c00768 Jonathan L Chen 1 , Peiyuan Zhang 1 , Masahito Abe 1 , Haruo Aikawa 1 , Liying Zhang 2 , Alexander J Frank 3 , Timothy Zembryski 3 , Christopher Hubbs 1 , HaJeung Park 1 , Jane Withka 2 , Claire Steppan 4 , Lucy Rogers 4 , Shawn Cabral 4 , Martin Pettersson 2 , Travis T Wager 2 , Matthew A Fountain 3 , Gavin Rumbaugh 1 , Jessica L Childs-Disney 1 , Matthew D Disney 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-05-04 , DOI: 10.1021/jacs.0c00768 Jonathan L Chen 1 , Peiyuan Zhang 1 , Masahito Abe 1 , Haruo Aikawa 1 , Liying Zhang 2 , Alexander J Frank 3 , Timothy Zembryski 3 , Christopher Hubbs 1 , HaJeung Park 1 , Jane Withka 2 , Claire Steppan 4 , Lucy Rogers 4 , Shawn Cabral 4 , Martin Pettersson 2 , Travis T Wager 2 , Matthew A Fountain 3 , Gavin Rumbaugh 1 , Jessica L Childs-Disney 1 , Matthew D Disney 1
Affiliation
|
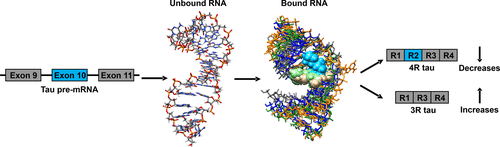
Approximately 95% of human genes are alternatively spliced, and aberrant splicing events can cause disease. One pre-mRNA that is alternatively spliced and linked to neurodegenerative diseases is tau (microtubule-associated protein tau), which can cause frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) and can contribute to Alzheimer's disease. Here, we describe the design of structure-specific lead small molecules that directly target tau pre-mRNA from sequence. This was followed by hit expansion and analogue synthesis to further improve upon these initial lead molecules. The emergent compounds were assessed for functional activity in a battery of assays, including binding assays and an assay that mimics molecular recognition of tau pre-mRNA by a U1 small nuclear ribonucleoprotein (snRNP) splicing factor. Compounds that emerged from these studies had enhanced potency and selectivity for the target RNA relative to the initial hits, while also having significantly improved drug-like properties. The compounds are shown to directly target tau pre-mRNA in cells, via chemical cross-linking and isolation by pull-down target profiling, and to rescue disease-relevant splicing of tau pre-mRNA in a variety of cellular systems, including primary neurons. More broadly, this study shows that lead, structure-specific compounds can be designed from sequence and then further optimized for their physicochemical properties while at the same time enhancing their activity.
中文翻译:

靶向 Tau mRNA 前体并影响剪接的小分子的设计、优化和研究
大约 95% 的人类基因是选择性剪接的,异常剪接事件可能导致疾病。tau(微管相关蛋白 tau)是一种选择性剪接并与神经退行性疾病相关的前 mRNA,它可导致额颞叶痴呆和与 17 号染色体 (FTDP-17) 相关的帕金森病,并可能导致阿尔茨海默病。在这里,我们描述了结构特异性先导小分子的设计,该小分子直接靶向序列中的 tau 前体 mRNA。接下来是命中扩展和类似物合成,以进一步改进这些初始先导分子。在一系列测定中评估了新出现的化合物的功能活性,包括结合测定和模拟 U1 小核核糖核蛋白 (snRNP) 剪接因子对 tau 前体 mRNA 的分子识别的测定。这些研究中出现的化合物相对于最初的命中增强了对靶标 RNA 的效力和选择性,同时还具有显着改善的药物样特性。这些化合物通过化学交联和下拉靶标分析分离,直接靶向细胞中的 tau 前体 mRNA,并在多种细胞系统(包括原代神经元)中挽救与疾病相关的 tau 前体 mRNA 剪接。更广泛地说,这项研究表明,可以根据序列设计先导的结构特异性化合物,然后进一步优化其理化性质,同时增强其活性。
更新日期:2020-05-04
中文翻译:

靶向 Tau mRNA 前体并影响剪接的小分子的设计、优化和研究
大约 95% 的人类基因是选择性剪接的,异常剪接事件可能导致疾病。tau(微管相关蛋白 tau)是一种选择性剪接并与神经退行性疾病相关的前 mRNA,它可导致额颞叶痴呆和与 17 号染色体 (FTDP-17) 相关的帕金森病,并可能导致阿尔茨海默病。在这里,我们描述了结构特异性先导小分子的设计,该小分子直接靶向序列中的 tau 前体 mRNA。接下来是命中扩展和类似物合成,以进一步改进这些初始先导分子。在一系列测定中评估了新出现的化合物的功能活性,包括结合测定和模拟 U1 小核核糖核蛋白 (snRNP) 剪接因子对 tau 前体 mRNA 的分子识别的测定。这些研究中出现的化合物相对于最初的命中增强了对靶标 RNA 的效力和选择性,同时还具有显着改善的药物样特性。这些化合物通过化学交联和下拉靶标分析分离,直接靶向细胞中的 tau 前体 mRNA,并在多种细胞系统(包括原代神经元)中挽救与疾病相关的 tau 前体 mRNA 剪接。更广泛地说,这项研究表明,可以根据序列设计先导的结构特异性化合物,然后进一步优化其理化性质,同时增强其活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号