当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Partial Oxidation of Ethanol in Supercritical Water
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-05-04 , DOI: 10.1021/acs.iecr.0c00945 Brian R. Pinkard 1 , Anmol L. Purohit 1 , Stuart J. Moore 1 , John C. Kramlich 1 , Per G. Reinhall 1 , Igor V. Novosselov 1, 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-05-04 , DOI: 10.1021/acs.iecr.0c00945 Brian R. Pinkard 1 , Anmol L. Purohit 1 , Stuart J. Moore 1 , John C. Kramlich 1 , Per G. Reinhall 1 , Igor V. Novosselov 1, 2
Affiliation
|
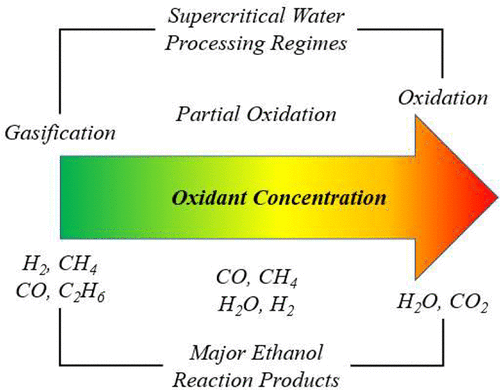
Ethanol is partially oxidized in a continuous supercritical water reactor at temperatures from 500 to 530 °C, constant pressure of 25 MPa, initial ethanol concentration of 5 wt %, residence times of 3–8 s, and oxidant-to-fuel stoichiometric equivalence ratios of 5, 7.5, and 10%. The experimental conditions are selected to study the regime where ethanol oxidation happens rapidly but below the temperature necessary to initiate hydrolysis reactions. The reactions and interactions of intermediate species can be analyzed, leveraging previous experimental results and the existing body of literature on ethanol hydrolysis, pyrolysis, and oxidation. Higher oxidant concentration increases ethanol destruction and gasification efficiency, although significant coke/char buildup is qualitatively observed within the reactor. Product yields from the experiments are used to infer significant reaction mechanisms, and a pathway is postulated for the counterintuitive formation of char under the studied conditions.
中文翻译:

超临界水中乙醇的部分氧化
乙醇在温度为500至530°C,恒定压力为25 MPa,初始乙醇浓度为5 wt%,停留时间为3-8 s,氧化剂与燃料的化学计量当量比的连续超临界水反应器中被部分氧化5、7.5和10%。选择实验条件以研究乙醇氧化迅速发生但低于引发水解反应所需温度的条件。可以利用先前的实验结果和有关乙醇水解,热解和氧化的现有文献,来分析中间物种的反应和相互作用。较高的氧化剂浓度增加了乙醇的破坏和气化效率,尽管在反应器中定性观察到大量的焦炭/焦炭积聚。
更新日期:2020-05-04
中文翻译:

超临界水中乙醇的部分氧化
乙醇在温度为500至530°C,恒定压力为25 MPa,初始乙醇浓度为5 wt%,停留时间为3-8 s,氧化剂与燃料的化学计量当量比的连续超临界水反应器中被部分氧化5、7.5和10%。选择实验条件以研究乙醇氧化迅速发生但低于引发水解反应所需温度的条件。可以利用先前的实验结果和有关乙醇水解,热解和氧化的现有文献,来分析中间物种的反应和相互作用。较高的氧化剂浓度增加了乙醇的破坏和气化效率,尽管在反应器中定性观察到大量的焦炭/焦炭积聚。

































 京公网安备 11010802027423号
京公网安备 11010802027423号