当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Predicting Spinel Disorder and Its Effect on Oxygen Transport Kinetics in Hercynite.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-05-04 , DOI: 10.1021/acsami.0c02819 Ryan M Trottier 1 , Zachary J L Bare 1 , Samantha L Millican 1 , Charles B Musgrave 1, 2, 3, 4
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-05-04 , DOI: 10.1021/acsami.0c02819 Ryan M Trottier 1 , Zachary J L Bare 1 , Samantha L Millican 1 , Charles B Musgrave 1, 2, 3, 4
Affiliation
|
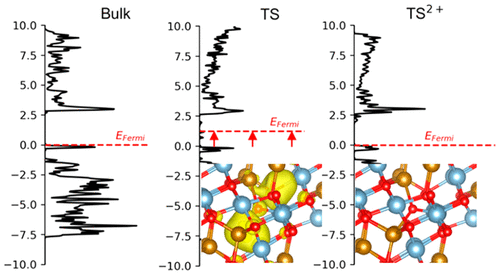
The iron aluminate spinel hercynite (FeAl2O4) is a promising redox material for solar thermochemical hydrogen production (STCH). Although it has a high H2 production capacity, the kinetics of its oxidation and reduction may be too slow to be practical for STCH. However, our results suggest that Fe-rich hercynite may have substantially faster redox kinetics, which could make hercynite competitive with other materials for STCH. We used density functional theory to investigate the origin of hercynite's slow kinetic behavior and show that it arises from the high activation barrier of 2.46 eV for oxygen vacancy (VO) diffusion in normal hercynite. To model the effect of disorder caused by spinel inversion, we examined 11 of the most common cation arrangements and found a near 1:1 correlation between the diffusion barrier and VO formation energy, both of which decrease by 0.6 eV for each additional nearest-neighbor Fe atom. To examine this trend, we used integrated crystal orbital Hamilton population (ICOHP) analysis to estimate the difference in the metal-oxygen bond strengths of cations neighboring VO and the diffusion transition state. The ICOHP predicted bond strengths correlate to both the diffusion barrier and VO formation energy. We also computed the effect of the charge state of the oxygen vacancy and found that positively charged vacancies are stable at low Fermi energies and have a diffusion barrier of only 0.79 eV, 1.67 eV lower than that of the neutral vacancy, demonstrating that stabilizing these charged vacancies may enable faster oxidation and reduction kinetics in hercynite. We show that uncompensated Fe antisite defects, which are present in Fe-rich hercynite, provide redox flexibility that stabilizes the charged VO and thereby increase the rate of VO diffusion. Finally, we predict that at higher VO concentrations the diffusion barrier depends on the relative positions of the vacancies and decreases when they are next-nearest neighbors.
中文翻译:

预测尖晶石紊乱及其对海藻土中氧输运动力学的影响。
铝酸铁尖晶石锂铁矿(FeAl2O4)是用于太阳能热化学制氢(STCH)的有前途的氧化还原材料。尽管它具有很高的氢气生产能力,但其氧化和还原的动力学可能太慢,以至于STCH都不实用。但是,我们的结果表明,富铁的锂铁矿可能具有明显更快的氧化还原动力学,这可能使锂铁矿与STCH的其他材料竞争。我们使用密度泛函理论研究了海藻土慢动力学行为的起源,并表明它是由2.46 eV的高活化势垒引起的,该活化障碍是正常海藻土中氧空位(VO)扩散的原因。为了模拟尖晶石倒置引起的无序效应,我们检查了11种最常见的阳离子排列,发现扩散势垒与VO形成能之间存在接近1:1的相关性,对于每一个额外的最近邻的Fe原子,两者都降低了0.6 eV。为了检查这种趋势,我们使用了集成的晶体轨道汉密尔顿人口(ICOHP)分析来估计与VO相邻的阳离子的金属-氧键强度和扩散过渡态的差异。ICOHP预测的结合强度与扩散势垒和VO形成能相关。我们还计算了氧空位的电荷状态的影响,发现带正电的空位在低费米能下是稳定的,其扩散势垒仅为0.79 eV,比中性空位低1.67 eV,表明稳定了这些空位空位可以使锂铁矿更快地氧化和还原动力学。我们证明了在富铁的锂锰矿中存在未补偿的铁反位缺陷,提供氧化还原柔韧性,可稳定带电的VO,从而提高VO扩散速率。最后,我们预测,在较高的VO浓度下,扩散壁垒取决于空位的相对位置,并且当它们是下一个最近的邻居时会减小。
更新日期:2020-05-04
中文翻译:

预测尖晶石紊乱及其对海藻土中氧输运动力学的影响。
铝酸铁尖晶石锂铁矿(FeAl2O4)是用于太阳能热化学制氢(STCH)的有前途的氧化还原材料。尽管它具有很高的氢气生产能力,但其氧化和还原的动力学可能太慢,以至于STCH都不实用。但是,我们的结果表明,富铁的锂铁矿可能具有明显更快的氧化还原动力学,这可能使锂铁矿与STCH的其他材料竞争。我们使用密度泛函理论研究了海藻土慢动力学行为的起源,并表明它是由2.46 eV的高活化势垒引起的,该活化障碍是正常海藻土中氧空位(VO)扩散的原因。为了模拟尖晶石倒置引起的无序效应,我们检查了11种最常见的阳离子排列,发现扩散势垒与VO形成能之间存在接近1:1的相关性,对于每一个额外的最近邻的Fe原子,两者都降低了0.6 eV。为了检查这种趋势,我们使用了集成的晶体轨道汉密尔顿人口(ICOHP)分析来估计与VO相邻的阳离子的金属-氧键强度和扩散过渡态的差异。ICOHP预测的结合强度与扩散势垒和VO形成能相关。我们还计算了氧空位的电荷状态的影响,发现带正电的空位在低费米能下是稳定的,其扩散势垒仅为0.79 eV,比中性空位低1.67 eV,表明稳定了这些空位空位可以使锂铁矿更快地氧化和还原动力学。我们证明了在富铁的锂锰矿中存在未补偿的铁反位缺陷,提供氧化还原柔韧性,可稳定带电的VO,从而提高VO扩散速率。最后,我们预测,在较高的VO浓度下,扩散壁垒取决于空位的相对位置,并且当它们是下一个最近的邻居时会减小。































 京公网安备 11010802027423号
京公网安备 11010802027423号