Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improving the Catalytic Activity of Carbon-Supported Single Atom Catalysts by Polynary Metal or Heteroatom Doping.
Small ( IF 13.0 ) Pub Date : 2020-05-04 , DOI: 10.1002/smll.201906782 Mengmeng Fan 1, 2 , Jiewu Cui 3 , Jingjie Wu 4 , Robert Vajtai 2, 5 , Dongping Sun 6 , Pulickel M Ajayan 2
Small ( IF 13.0 ) Pub Date : 2020-05-04 , DOI: 10.1002/smll.201906782 Mengmeng Fan 1, 2 , Jiewu Cui 3 , Jingjie Wu 4 , Robert Vajtai 2, 5 , Dongping Sun 6 , Pulickel M Ajayan 2
Affiliation
|
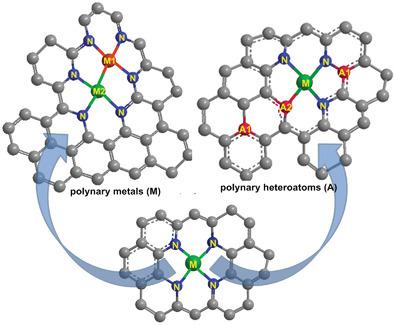
Single atom catalysts (SACs) are widely researched in various chemical transformations due to the high atomic utilization and catalytic activity. Carbon‐supported SACs are the largest class because of the many excellent properties of carbon derivatives. The single metal atoms are usually immobilized by doped N atoms and in some cases by C geometrical defects on carbon materials. To explore the catalytic mechanisms and improve the catalytic performance, many efforts have been devoted to modulating the electronic structure of metal single atomic sites. Doping with polynary metals and heteroatoms has been recently proposed to be a simple and effective strategy, derived from the modulating mechanisms of metal alloy structure for metal catalysts and from the donating/withdrawing heteroatom doping for carbon supports, respectively. Polynary metals SACs involve two types of metal with atomical dispersion. The bimetal atom pairs act as dual catalytic sites leading to higher catalytic activity and selectivity. Polynary heteroatoms generally have two types of heteroatoms in which N always couples with another heteroatom, including B, S, P, etc. In this Review, the recent progress of polynary metals and heteroatoms SACs is summarized. Finally, the barriers to tune the activity/selectivity of SACs are discussed and further perspectives presented.
中文翻译:

通过多元金属或杂原子掺杂提高碳载单原子催化剂的催化活性。
由于高的原子利用率和催化活性,单原子催化剂(SAC)在各种化学转化中得到了广泛的研究。由于碳衍生物的许多优异性能,碳支持的SAC是最大的类别。单个金属原子通常被掺杂的N原子固定,在某些情况下,被碳材料上的C几何缺陷固定。为了探索催化机理和提高催化性能,已致力于调节金属单原子位的电子结构。近年来,从多元金属催化剂的金属合金结构的调节机理和碳载体的供体/撤出杂原子的掺杂中,提出了多元金属和杂原子的掺杂是一种简单有效的策略。多元金属SAC涉及两种原子分散的金属。双金属原子对充当双重催化位点,导致更高的催化活性和选择性。多元杂原子通常具有两种类型的杂原子,其中N总是与另一个杂原子偶合,包括B,S,P等。在本综述中,总结了多元金属和杂原子SAC的最新进展。最后,讨论了调节SAC活性/选择性的障碍,并提出了其他观点。综述了多元金属和杂原子SAC的最新进展。最后,讨论了调节SAC活性/选择性的障碍,并提出了其他观点。综述了多元金属和杂原子SAC的最新进展。最后,讨论了调节SAC活性/选择性的障碍,并提出了其他观点。
更新日期:2020-05-04
中文翻译:

通过多元金属或杂原子掺杂提高碳载单原子催化剂的催化活性。
由于高的原子利用率和催化活性,单原子催化剂(SAC)在各种化学转化中得到了广泛的研究。由于碳衍生物的许多优异性能,碳支持的SAC是最大的类别。单个金属原子通常被掺杂的N原子固定,在某些情况下,被碳材料上的C几何缺陷固定。为了探索催化机理和提高催化性能,已致力于调节金属单原子位的电子结构。近年来,从多元金属催化剂的金属合金结构的调节机理和碳载体的供体/撤出杂原子的掺杂中,提出了多元金属和杂原子的掺杂是一种简单有效的策略。多元金属SAC涉及两种原子分散的金属。双金属原子对充当双重催化位点,导致更高的催化活性和选择性。多元杂原子通常具有两种类型的杂原子,其中N总是与另一个杂原子偶合,包括B,S,P等。在本综述中,总结了多元金属和杂原子SAC的最新进展。最后,讨论了调节SAC活性/选择性的障碍,并提出了其他观点。综述了多元金属和杂原子SAC的最新进展。最后,讨论了调节SAC活性/选择性的障碍,并提出了其他观点。综述了多元金属和杂原子SAC的最新进展。最后,讨论了调节SAC活性/选择性的障碍,并提出了其他观点。






























 京公网安备 11010802027423号
京公网安备 11010802027423号