当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Syntheses of (-)-Strictosidine and Related Indole Alkaloid Glycosides.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-04 , DOI: 10.1002/anie.202005748 Jukiya Sakamoto 1 , Yuhei Umeda 1 , Kenta Rakumitsu 1 , Michinori Sumimoto 2 , Hayato Ishikawa 1, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-04 , DOI: 10.1002/anie.202005748 Jukiya Sakamoto 1 , Yuhei Umeda 1 , Kenta Rakumitsu 1 , Michinori Sumimoto 2 , Hayato Ishikawa 1, 3
Affiliation
|
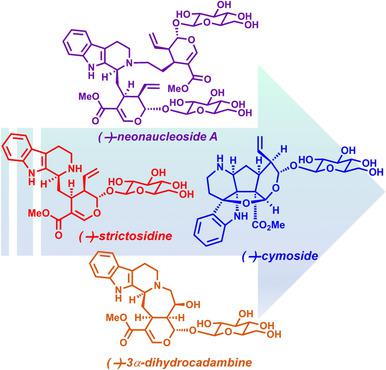
A collective synthesis of glycosylated monoterpenoid indole alkaloids is reported. A highly diastereoselective Pictet–Spengler reaction with α‐cyanotryptamine and secologanin tetraacetate as substrates, followed by a reductive decyanation reaction, was developed for the synthesis of (−)‐strictosidine, which is an important intermediate in biosynthesis. This two‐step chemical method was established as an alternative to the biosynthetically employed strictosidine synthase. Furthermore, after carrying out chemical and computational studies, a transition state for induction of diastereoselectivity in our newly discovered Pictet–Spengler reaction is proposed. Having achieved the first enantioselective total synthesis of (−)‐strictosidine in just 10 steps, subsequent bioinspired transformations resulted in the concise total syntheses of (−)‐strictosamide, (−)‐neonaucleoside A, (−)‐cymoside, and (−)‐3α‐dihydrocadambine.
中文翻译:

(-)-Strictosidine和相关的吲哚生物碱糖苷的总合成。
据报道糖基化的单萜类吲哚生物碱的集体合成。开发了一种高度非对映选择性的Pictet-Spengler反应,以α-氰基色胺和secologanin四乙酸酯为底物,然后进行还原性脱氰反应,用于合成(-)-strictosidine,这是生物合成的重要中间体。建立了此两步化学方法,替代了生物合成采用的严格的芥子碱合酶。此外,在进行化学和计算研究之后,提出了在我们新发现的Pictet-Spengler反应中诱导非对映选择性的过渡态。仅用10个步骤就完成了(-)-strictosidine的首次对映选择性全合成,
更新日期:2020-05-04
中文翻译:

(-)-Strictosidine和相关的吲哚生物碱糖苷的总合成。
据报道糖基化的单萜类吲哚生物碱的集体合成。开发了一种高度非对映选择性的Pictet-Spengler反应,以α-氰基色胺和secologanin四乙酸酯为底物,然后进行还原性脱氰反应,用于合成(-)-strictosidine,这是生物合成的重要中间体。建立了此两步化学方法,替代了生物合成采用的严格的芥子碱合酶。此外,在进行化学和计算研究之后,提出了在我们新发现的Pictet-Spengler反应中诱导非对映选择性的过渡态。仅用10个步骤就完成了(-)-strictosidine的首次对映选择性全合成,


















































 京公网安备 11010802027423号
京公网安备 11010802027423号