当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Ir/Ni(OH)2 Heterostructured Electrocatalyst for the Oxygen Evolution Reaction: Breaking the Scaling Relation, Stabilizing Iridium(V), and Beyond.
Advanced Materials ( IF 27.4 ) Pub Date : 2020-05-04 , DOI: 10.1002/adma.202000872
Guoqiang Zhao 1, 2 , Peng Li 2 , Ningyan Cheng 2 , Shi Xue Dou 2 , Wenping Sun 1, 2
Advanced Materials ( IF 27.4 ) Pub Date : 2020-05-04 , DOI: 10.1002/adma.202000872
Guoqiang Zhao 1, 2 , Peng Li 2 , Ningyan Cheng 2 , Shi Xue Dou 2 , Wenping Sun 1, 2
Affiliation
|
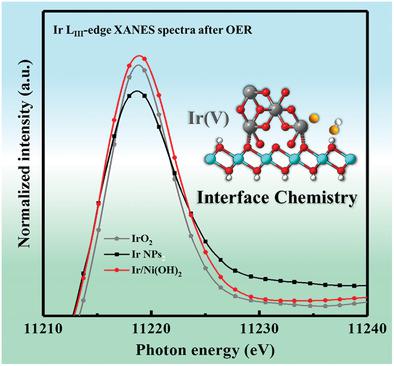
Developing efficient electrocatalysts for the oxygen evolution reaction (OER) is highly challenging for hydrogen production from water splitting, due to the high energy barrier for OO bond formation and the restriction of the scaling relation between the multiple reaction intermediates. In order to simultaneously address these concerns, an Ir/Ni(OH)2 heterostructure with abundant heterointerfaces is deliberately designed as an efficient electrocatalyst system, with Ir nanoparticles (NPs) homogeneously confined on the Ni(OH)2 nanosheets. The strong electronic interaction and chemical bonding across the interface between the Ir and Ni(OH)2 can effectively stabilize the metastable electrophilic Ir(V) species, which is vital to boost the formation of OO bonds. Meanwhile, the adsorption of the multiple intermediates is synergistically optimized at the heterointerface, which breaks the restrictive scaling relation and substantially accelerates the OER kinetics. In addition, the severe agglomeration of Ir species is greatly mitigated by the confinement effect, ensuring the structural integrity of the catalyst and the constant exposure of active sites. Owing to its well‐defined multifunctional interfaces, the Ir/Ni(OH)2 heterostructure exhibits exceptional OER activity and durability in alkaline media. The present results highlight the significance of heterostructure interface engineering toward the rational design and development of advanced electrocatalysts for the OER and beyond.
中文翻译:

用于氧气析出反应的Ir / Ni(OH)2异质结构电催化剂:打破结垢关系,稳定铱(V)等。
由于OO键形成的高能垒和多种反应中间体之间结垢关系的限制,开发用于氧分解反应(OER)的高效电催化剂对水分解产生氢气的挑战很大。为了同时解决这些问题,故意将具有丰富异质界面的Ir / Ni(OH)2异质结构设计为一种有效的电催化剂体系,将Ir纳米颗粒(NP)均匀地限制在Ni(OH)2纳米片上。Ir和Ni(OH)2之间的界面具有很强的电子相互作用和化学键可以有效地稳定亚稳的亲电Ir(V)物种,这对于促进OO键的形成至关重要。同时,在异质界面上协同优化了多种中间体的吸附,这打破了限制性的比例关系,并大大促进了OER动力学。此外,限制效应极大地减轻了Ir物种的严重团聚,确保了催化剂的结构完整性和活性位点的持续暴露。由于其定义明确的多功能接口,Ir / Ni(OH)2异质结构在碱性介质中具有出色的OER活性和耐久性。目前的结果凸显了异质结构界面工程对于OER及其它以后的先进电催化剂的合理设计和开发的重要性。
更新日期:2020-05-04
中文翻译:

用于氧气析出反应的Ir / Ni(OH)2异质结构电催化剂:打破结垢关系,稳定铱(V)等。
由于OO键形成的高能垒和多种反应中间体之间结垢关系的限制,开发用于氧分解反应(OER)的高效电催化剂对水分解产生氢气的挑战很大。为了同时解决这些问题,故意将具有丰富异质界面的Ir / Ni(OH)2异质结构设计为一种有效的电催化剂体系,将Ir纳米颗粒(NP)均匀地限制在Ni(OH)2纳米片上。Ir和Ni(OH)2之间的界面具有很强的电子相互作用和化学键可以有效地稳定亚稳的亲电Ir(V)物种,这对于促进OO键的形成至关重要。同时,在异质界面上协同优化了多种中间体的吸附,这打破了限制性的比例关系,并大大促进了OER动力学。此外,限制效应极大地减轻了Ir物种的严重团聚,确保了催化剂的结构完整性和活性位点的持续暴露。由于其定义明确的多功能接口,Ir / Ni(OH)2异质结构在碱性介质中具有出色的OER活性和耐久性。目前的结果凸显了异质结构界面工程对于OER及其它以后的先进电催化剂的合理设计和开发的重要性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号