International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2020-05-04 , DOI: 10.1016/j.ijhydene.2020.04.046 Xinran Zhao , Xiaobo He , Fengxiang Yin , Biaohua Chen , Guoru Li , Huaqiang Yin
|
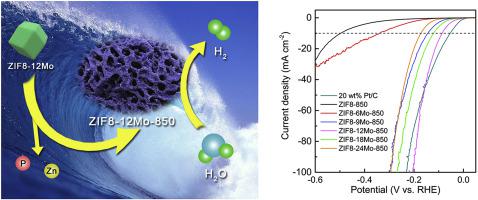
A metal-organic framework-derived method was developed to synthesize highly efficient non-noble metal electrocatalyst for alkaline hydrogen evolution reaction (HER). Zn2+, phosphomolybdic acid were coordinated with 2-methylimidazole, and zinc (Zn) and phosphorus (P) species were removed by annealing at 850 °C in N2 atmosphere, resulting in micro/mesoporous molybdenum carbide (Mo2C) composited with nitrogen-doped carbon (denoted as ZIF8-xMo-850). The optimized sample ZIF8-12Mo-850 displayed a low overpotential of ~85.7 mV to deliver a current density of 10 mA cm−2, with a corresponding Tafel slope of ~69.7 mV dec−1 in 1 M KOH. This HER catalytic activity was competitive with the most recently developed Mo2C-based HER electrocatalysts. From further investigation, the high HER catalytic activity of ZIF8-12Mo-850 is owing to three aspects: (i) The appropriate Mo feeding amount of ZIF8-12Mo-850 resulted in the highest surface content of Mo2+ active site; (ii) The evaporation of Zn and P in the ZIF8-12Mo precursor formed its largest average pore diameter of 32.3 nm, which leaded to the highest electrochemically active surface area (ECSA) of 64.29 cm2 (iii) The 2-methylimidazole in the precursor resulted in the highest surface content of pyridinic N in ZIF8-12Mo-850 (14.63%), which efficiently improved its conductivity and charge transfer efficiency.
中文翻译:

沸石咪唑啉骨架8衍生的碳化钼/氮掺杂碳可用于高效放氢反应
开发了一种金属有机骨架的方法,以合成用于碱氢析出反应(HER)的高效非贵金属电催化剂。Zn 2+和磷钼酸与2-甲基咪唑配位,在N 2气氛中于850°C退火去除锌(Zn)和磷(P)物种,从而合成了微/中孔碳化钼(Mo 2 C)掺氮碳(表示为ZIF8-xMo-850)。经过优化的样品ZIF8-12Mo-850在1 M KOH中显示约85.7 mV的低过电势,可提供10 mA cm -2的电流密度,相应的Tafel斜率约为69.7 mV dec -1。这种HER催化活性与最新开发的Mo具有竞争性2 C基HER电催化剂。通过进一步的研究,ZIF8-12Mo-850的高HER催化活性归因于以下三个方面:(i)ZIF8-12Mo-850的适量Mo喂入导致Mo 2+活性位点的最高表面含量;(ii)ZIF8-12Mo前体中的Zn和P蒸发形成了最大的平均孔径32.3 nm,这导致了64.29 cm 2的最高电化学活性表面积(ECSA)(iii)前驱体导致ZIF8-12Mo-850中吡啶N的最高表面含量(14.63%),从而有效地提高了其电导率和电荷转移效率。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号