当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of new thieno[3,2-d]pyrimidine derivatives targeting EGFRL858R/T790M NSCLCs by the conformation constrained strategy.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-05-04 , DOI: 10.1016/j.ejmech.2020.112388 Yang Chen 1 , Linlin Yang 2 , Hui Qiao 1 , Zhongyu Cheng 1 , Jiahao Xie 1 , Wenjuan Zhou 1 , Xin Huang 1 , Yaoxuan Jiang 1 , Bin Yu 1 , Wen Zhao 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-05-04 , DOI: 10.1016/j.ejmech.2020.112388 Yang Chen 1 , Linlin Yang 2 , Hui Qiao 1 , Zhongyu Cheng 1 , Jiahao Xie 1 , Wenjuan Zhou 1 , Xin Huang 1 , Yaoxuan Jiang 1 , Bin Yu 1 , Wen Zhao 1
Affiliation
|
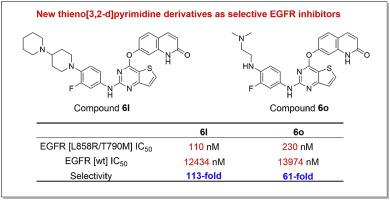
Studies on the third-generation of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) targeting EGFRL858R/T790M mutant remain hotspots, specifically for non-small cell lung cancer (NSCLC). In the current study, a new series of EGFR-TKIs with thieno[3,2-d]pyrimidine derivatives(6a-6r) bearing quinolin-2(1H)-ones were designed and synthesized, through conformational constrained strategy from the third generation of EGFR-TKI olmutinib. In vitro structure-activity relationship (SAR) studies indicated that compounds 6a, 6l, 6m, 6n and 6o exhibited good selective inhibition to EGFRL858R/T790M (IC50 ≤ 250 nM) over wild type EGFR (IC50 > 10000 nM). The observed selectivity of compounds 6l and 6o was also proved by the computational molecular docking and the cellular thermal shift assay. These compounds had good growth inhibitory effect on the four tested cancer cell lines. Specifically, 6o could significantly inhibit the colony formation, wound healing and the expression of p-EGFR and its downstream p-ERK in EGFRL858R/T790M H1975 lung cancer cells. Our findings suggest that the thieno[3,2-d]pyrimidine compounds, especially 6l and 6o, can selectively target the mutant EGFRL858R/T790M in vitro and at cellular level and may serve as the lead compounds for generating new series of the third-generation EGFR-TKIs.
中文翻译:

通过构象受限策略发现了靶向EGFRL858R / T790M NSCLC的新噻吩并[3,2-d]嘧啶衍生物。
针对EGFRL858R / T790M突变体的第三代表皮生长因子受体酪氨酸激酶抑制剂(EGFR-TKIs)的研究仍然是热点,特别是对于非小细胞肺癌(NSCLC)。在当前的研究中,通过第三代的构象约束策略,设计并合成了一系列新的带有噻吩[3,2-d]嘧啶衍生物(6a-6r)和喹啉-2(1H)-的EGFR-TKIs。 EGFR-TKI olmutinib的检测。体外结构活性关系(SAR)研究表明,化合物6a,6l,6m,6n和6o对EGFRL858R / T790M(IC50≤250 nM)的抑制作用优于野生型EGFR(IC50> 10000 nM)。化合物6l和6o的观察到的选择性也通过计算分子对接和细胞热位移测定法得以证明。这些化合物对四种测试的癌细胞系具有良好的生长抑制作用。具体而言,6o可以显着抑制EGFRL858R / T790M H1975肺癌细胞的集落形成,伤口愈合以及p-EGFR及其下游p-ERK的表达。我们的发现表明,噻吩并[3,2-d]嘧啶化合物,尤其是6l和6o,可以在体外和细胞水平上选择性靶向突变型EGFRL858R / T790M,并且可以作为产生第三系列新化合物的先导化合物代EGFR-TKIs。
更新日期:2020-05-04
中文翻译:

通过构象受限策略发现了靶向EGFRL858R / T790M NSCLC的新噻吩并[3,2-d]嘧啶衍生物。
针对EGFRL858R / T790M突变体的第三代表皮生长因子受体酪氨酸激酶抑制剂(EGFR-TKIs)的研究仍然是热点,特别是对于非小细胞肺癌(NSCLC)。在当前的研究中,通过第三代的构象约束策略,设计并合成了一系列新的带有噻吩[3,2-d]嘧啶衍生物(6a-6r)和喹啉-2(1H)-的EGFR-TKIs。 EGFR-TKI olmutinib的检测。体外结构活性关系(SAR)研究表明,化合物6a,6l,6m,6n和6o对EGFRL858R / T790M(IC50≤250 nM)的抑制作用优于野生型EGFR(IC50> 10000 nM)。化合物6l和6o的观察到的选择性也通过计算分子对接和细胞热位移测定法得以证明。这些化合物对四种测试的癌细胞系具有良好的生长抑制作用。具体而言,6o可以显着抑制EGFRL858R / T790M H1975肺癌细胞的集落形成,伤口愈合以及p-EGFR及其下游p-ERK的表达。我们的发现表明,噻吩并[3,2-d]嘧啶化合物,尤其是6l和6o,可以在体外和细胞水平上选择性靶向突变型EGFRL858R / T790M,并且可以作为产生第三系列新化合物的先导化合物代EGFR-TKIs。






























 京公网安备 11010802027423号
京公网安备 11010802027423号