当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of an Isothiazolinone-Containing Antitubercular Natural Product Levesquamide.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-05-04 , DOI: 10.1021/acs.joc.0c00339 Libang Liang , Bradley Haltli 1 , Douglas H Marchbank 1 , Maike Fischer 2 , Christopher W Kirby 2 , Hebelin Correa 1 , Trevor N Clark 3 , Christopher A Gray 3, 4 , Russell G Kerr 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-05-04 , DOI: 10.1021/acs.joc.0c00339 Libang Liang , Bradley Haltli 1 , Douglas H Marchbank 1 , Maike Fischer 2 , Christopher W Kirby 2 , Hebelin Correa 1 , Trevor N Clark 3 , Christopher A Gray 3, 4 , Russell G Kerr 1
Affiliation
|
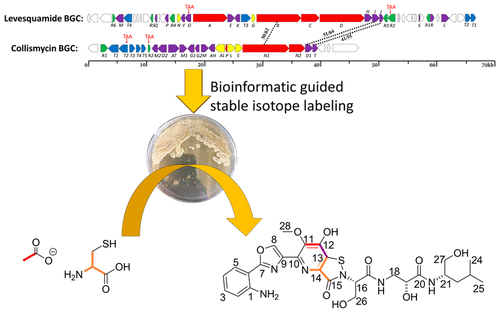
Antitubercular agent levesquamide is a new polyketide-nonribosomal peptide (PK-NRP) hybrid marine natural product isolated from Streptomyces sp. RKND-216. The structure contains a rare isothiazolinone moiety which has only been reported in collismycin SN. Structure elucidation by NMR spectroscopy was a significant challenge due to a deficiency of protons in this aromatic moiety. Therefore, the genome of Streptomyces sp. RKND-216 was sequenced to identify the levesquamide biosynthetic gene cluster (BGC). Analysis of the BGC provided structural insights and guided stable-isotope labeling experiments, which led to the assignment of the fused pyridine-isothiazolinone moiety. The BGC and the labeling experiments provide further insights into the biosynthetic origin of isothiazolinones. Levesquamide exhibited antimicrobial activity in the microplate alamarBlue assay (MABA) and low oxygen recovery assay (LORA) against Mycobacterium tuberculosis H37Rv with minimum inhibitory concentration (MIC) values of 9.65 and 22.28 μM, respectively. Similar activity was exhibited against rifampicin- and isoniazid-resistant M. tuberculosis strains with MIC values of 9.46 and 9.90 μM, respectively. This result suggests levesquamide has a different mode of action against M. tuberculosis compared to the two first-line antitubercular drugs rifampicin and isoniazid. Furthermore, levesquamide shows no cytotoxicity against the Vero cell line, suggesting it may have a useful therapeutic window.
中文翻译:

发现含有异噻唑啉酮的抗结核天然产物左乙酰胺。
抗结核药左乙磺酰胺是一种新的聚酮-非核糖体肽(PK-NRP)杂种海洋天然产物,从链霉菌种中分离。RKND-216。该结构包含罕见的异噻唑啉酮部分,仅在Collismycin SN中有报道。由于该芳族部分中质子的缺乏,通过NMR光谱阐明结构是一项重大挑战。因此,链霉菌的基因组。对RKND-216进行测序,以鉴定左乙磺酰胺生物合成基因簇(BGC)。BGC的分析提供了结构上的见识和指导的稳定同位素标记实验,从而导致了熔融的吡啶-异噻唑啉酮部分的分配。BGC和标记实验为异噻唑啉酮的生物合成来源提供了进一步的见解。左乙酰胺在微孔板alamarBlue分析(MABA)和低氧回收分析(LORA)中显示出对结核分枝杆菌H37Rv的抗菌活性,最低抑菌浓度(MIC)值分别为9.65和22.28μM。对耐利福平和异烟肼的结核分枝杆菌菌株表现出相似的活性,其MIC值分别为9.46和9.90μM。该结果表明,与两种一线抗结核药物利福平和异烟肼相比,左乙酰胺对结核分枝杆菌具有不同的作用方式。此外,左乙磺酰胺对Vero细胞系无细胞毒性,表明它可能具有有用的治疗窗口。65和22.28μM。对耐利福平和异烟肼的结核分枝杆菌菌株表现出相似的活性,其MIC值分别为9.46和9.90μM。该结果表明,与两种一线抗结核药物利福平和异烟肼相比,左乙酰胺对结核分枝杆菌具有不同的作用方式。此外,左乙磺酰胺对Vero细胞系无细胞毒性,表明它可能具有有用的治疗窗口。65和22.28μM。对耐利福平和异烟肼的结核分枝杆菌菌株表现出相似的活性,其MIC值分别为9.46和9.90μM。该结果表明,与两种一线抗结核药物利福平和异烟肼相比,左乙酰胺对结核分枝杆菌具有不同的作用方式。此外,左乙磺酰胺对Vero细胞系无细胞毒性,表明它可能具有有用的治疗窗口。
更新日期:2020-05-04
中文翻译:

发现含有异噻唑啉酮的抗结核天然产物左乙酰胺。
抗结核药左乙磺酰胺是一种新的聚酮-非核糖体肽(PK-NRP)杂种海洋天然产物,从链霉菌种中分离。RKND-216。该结构包含罕见的异噻唑啉酮部分,仅在Collismycin SN中有报道。由于该芳族部分中质子的缺乏,通过NMR光谱阐明结构是一项重大挑战。因此,链霉菌的基因组。对RKND-216进行测序,以鉴定左乙磺酰胺生物合成基因簇(BGC)。BGC的分析提供了结构上的见识和指导的稳定同位素标记实验,从而导致了熔融的吡啶-异噻唑啉酮部分的分配。BGC和标记实验为异噻唑啉酮的生物合成来源提供了进一步的见解。左乙酰胺在微孔板alamarBlue分析(MABA)和低氧回收分析(LORA)中显示出对结核分枝杆菌H37Rv的抗菌活性,最低抑菌浓度(MIC)值分别为9.65和22.28μM。对耐利福平和异烟肼的结核分枝杆菌菌株表现出相似的活性,其MIC值分别为9.46和9.90μM。该结果表明,与两种一线抗结核药物利福平和异烟肼相比,左乙酰胺对结核分枝杆菌具有不同的作用方式。此外,左乙磺酰胺对Vero细胞系无细胞毒性,表明它可能具有有用的治疗窗口。65和22.28μM。对耐利福平和异烟肼的结核分枝杆菌菌株表现出相似的活性,其MIC值分别为9.46和9.90μM。该结果表明,与两种一线抗结核药物利福平和异烟肼相比,左乙酰胺对结核分枝杆菌具有不同的作用方式。此外,左乙磺酰胺对Vero细胞系无细胞毒性,表明它可能具有有用的治疗窗口。65和22.28μM。对耐利福平和异烟肼的结核分枝杆菌菌株表现出相似的活性,其MIC值分别为9.46和9.90μM。该结果表明,与两种一线抗结核药物利福平和异烟肼相比,左乙酰胺对结核分枝杆菌具有不同的作用方式。此外,左乙磺酰胺对Vero细胞系无细胞毒性,表明它可能具有有用的治疗窗口。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号