当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Insecticidal Activity of Novel Doramectin Derivatives Containing Acylurea and Acylthiourea Based on Hydrogen Bonding.
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-05-01 , DOI: 10.1021/acs.jafc.0c00230 Qi Zhang 1, 2 , Yao Cheng 1, 3 , Cheng Zheng 1, 3 , Ping Bai 1, 3 , Jian Yang 1, 3 , Xiaoxia Lu 1, 3
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-05-01 , DOI: 10.1021/acs.jafc.0c00230 Qi Zhang 1, 2 , Yao Cheng 1, 3 , Cheng Zheng 1, 3 , Ping Bai 1, 3 , Jian Yang 1, 3 , Xiaoxia Lu 1, 3
Affiliation
|
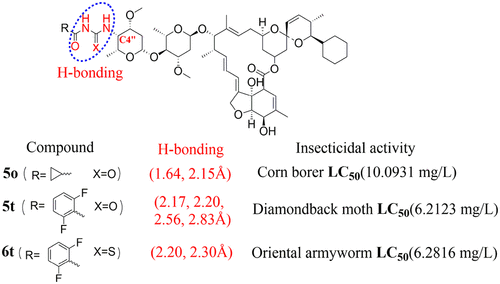
Our recent investigation on the insecticidal activities of several doramectin derivatives preliminarily revealed that the presence of hydrogen bonds at the C4″ position of the molecule with target protein γ-aminobutyric acid (GABA) receptor was crucial for retaining high insecticidal activity. As a continuation of our research work on the development of new insecticides, two series of novel acylurea and acylthiourea doramectin derivatives were designed and synthesized. The bioassay results indicated that the newly synthesized compounds (5o, 5t, and 6t) exhibited higher insecticidal activity against diamondback moth, oriental armyworm, and corn borer than the control compounds doramectin, commercial avermectins, chlorbenzuron, and lead compound 3g in our laboratory. Specifically, compound 5t was identified as the most promising insecticide against diamondback moth, with a final mortality rate of 80.00% at the low concentration of 12.50 mg/L, showing approximately 7.75-fold higher potency than the parent doramectin (LC50 value of 48.1547 mg/L), 6.52-fold higher potency than commercial avermectins (LC50 value of 40.5507 mg/L), and 3.98-fold higher potency than compound 3g (LC50 value of 24.7742 mg/L). Additionally, molecular docking simulations revealed that compound 5t (2.17, 2.20, 2.56, and 2.83 Å) displayed stronger hydrogen-bond action in binding with the GABA receptor, better than that of compound 5o (1.64 and 2.15 Å) and compound 6t (2.20 and 2.31 Å) at the C4″ position. This work demonstrated that these compounds containing hydrogen-bond groups might contribute to the improvement of insecticidal activity and supply certain hints toward structure optimization design for the development of new insecticides.
中文翻译:

基于氢键的含酰基脲和酰基硫脲的新型多拉菌素衍生物的设计,合成和杀虫活性。
我们最近对几种多拉菌素衍生物的杀虫活性的研究初步表明,在具有目标蛋白γ-氨基丁酸(GABA)受体的分子的C4''位置存在氢键对于保持高杀虫活性至关重要。作为我们对新型杀虫剂开发的研究工作的继续,设计并合成了两个系列的新型酰基脲和酰基硫脲多拉菌素衍生物。生物测定结果表明,在我们的实验室中,新合成的化合物(5o,5t和6t)对小菜蛾,东方粘虫和玉米bore的杀虫活性高于对照化合物doramectin,市售阿维菌素,敌百虫和铅化合物3g。特别,化合物5t被确定为最有前景的小菜蛾杀虫剂,在低浓度12.50 mg / L时最终死亡率为80.00%,其效力比母体多拉菌素高约7.75倍(LC50值为48.1547 mg / L ),效力比市售阿维菌素(LC50值为40.5507 mg / L)高6.52倍,效力比化合物3g(LC50值为24.7742 mg / L)高3.98倍。此外,分子对接模拟显示化合物5t(2.17、2.20、2.56和2.83Å)在与GABA受体结合中表现出更强的氢键作用,优于化合物5o(1.64和2.15Å)和化合物6t(2.20)在C4“位置的2.31Å)。
更新日期:2020-05-01
中文翻译:

基于氢键的含酰基脲和酰基硫脲的新型多拉菌素衍生物的设计,合成和杀虫活性。
我们最近对几种多拉菌素衍生物的杀虫活性的研究初步表明,在具有目标蛋白γ-氨基丁酸(GABA)受体的分子的C4''位置存在氢键对于保持高杀虫活性至关重要。作为我们对新型杀虫剂开发的研究工作的继续,设计并合成了两个系列的新型酰基脲和酰基硫脲多拉菌素衍生物。生物测定结果表明,在我们的实验室中,新合成的化合物(5o,5t和6t)对小菜蛾,东方粘虫和玉米bore的杀虫活性高于对照化合物doramectin,市售阿维菌素,敌百虫和铅化合物3g。特别,化合物5t被确定为最有前景的小菜蛾杀虫剂,在低浓度12.50 mg / L时最终死亡率为80.00%,其效力比母体多拉菌素高约7.75倍(LC50值为48.1547 mg / L ),效力比市售阿维菌素(LC50值为40.5507 mg / L)高6.52倍,效力比化合物3g(LC50值为24.7742 mg / L)高3.98倍。此外,分子对接模拟显示化合物5t(2.17、2.20、2.56和2.83Å)在与GABA受体结合中表现出更强的氢键作用,优于化合物5o(1.64和2.15Å)和化合物6t(2.20)在C4“位置的2.31Å)。































 京公网安备 11010802027423号
京公网安备 11010802027423号