当前位置:
X-MOL 学术
›
J. Mol. Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational landscape and intricate conformational relaxation paths of 4,4,4-trifluoro-1-butanol: Rotational spectroscopy and quantum chemical calculations
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.molstruc.2020.128359 Tao Lu , Fan Xie , Nathan A. Seifert , Wolfgang Jäger , Yunjie Xu
Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.molstruc.2020.128359 Tao Lu , Fan Xie , Nathan A. Seifert , Wolfgang Jäger , Yunjie Xu
|
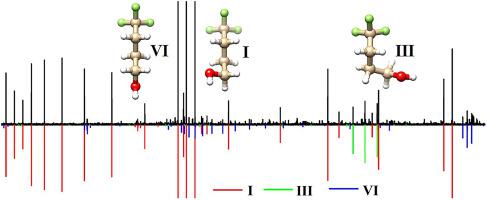
Abstract Fluorine-containing aliphatic alcohols have attracted considerable interest in part because they are widely used as solvents for peptides and proteins and for some chemical reactions. In the present work, the rotational spectrum of 4,4,4-trifluoro-1-butanol was investigated using a chirped-pulse Fourier transform microwave spectrometer in combination with a pulsed molecular expansion. The long carbon chain and the OH group in 4,4,4-trifluoro-1-butanol afford substantial conformational flexibility. In total, 27 possible geometries were identified using a semiempirical tight-binding based quantum chemistry program and also by carrying out systematic manual conformational searches. These conformations were verified to be true minima at the B3LYP-D3(BJ)/def2-TZVP, B3LYP-D3(BJ)/6–311++G(2d,p) and MP2/6–311++G(2d,p) levels of theory. 14 conformers that are spectroscopically distinguishable were obtained within an energy window of 18 kJ mol−1. Rotational spectra of three conformers were assigned, including four 13C isotopologues and one 18O isotopologue of the most stable conformer in their natural abundances. One of the detected conformers exhibits an intramolecular hydrogen bond formed between a non-hydroxy hydrogen atom of the –CH2OH group and a fluorine atom of the –CF3 group, which was identified by a quantum theory of atoms in molecules (QTAIM) analysis. The intricate conformational relaxation pathways and the corresponding interconversion barriers were explored to rationalize the experimental relative abundances of the detected conformers.
中文翻译:

4,4,4-三氟-1-丁醇的构象景观和复杂的构象弛豫路径:旋转光谱和量子化学计算
摘要 含氟脂肪醇引起了相当大的兴趣,部分原因是它们被广泛用作肽和蛋白质的溶剂以及一些化学反应。在目前的工作中,使用啁啾脉冲傅里叶变换微波光谱仪结合脉冲分子膨胀研究了 4,4,4-三氟-1-丁醇的旋转光谱。4,4,4-三氟-1-丁醇中的长碳链和OH基团提供了显着的构象灵活性。使用基于紧束缚的半经验量子化学程序以及通过进行系统的手动构象搜索,总共确定了 27 种可能的几何形状。这些构象在 B3LYP-D3(BJ)/def2-TZVP、B3LYP-D3(BJ)/6–311++G(2d,p) 和 MP2/6–311++G(2d ,p) 理论水平。在 18 kJ mol-1 的能量窗口内获得了 14 个在光谱上可区分的构象异构体。分配了三种构象异构体的旋转光谱,包括天然丰度中最稳定构象异构体的四种 13C 同位素体和一种 18O 同位素体。其中一个检测到的构象异构体表现出在 –CH2OH 基团的非羟基氢原子和 –CF3 基团的氟原子之间形成的分子内氢键,这是通过分子中原子的量子理论 (QTAIM) 分析确定的。探索了复杂的构象弛豫途径和相应的相互转换障碍,以使检测到的构象异构体的实验相对丰度合理化。分配了三种构象异构体的旋转光谱,包括天然丰度中最稳定构象异构体的四种 13C 同位素体和一种 18O 同位素体。其中一个检测到的构象异构体表现出在 –CH2OH 基团的非羟基氢原子和 –CF3 基团的氟原子之间形成的分子内氢键,这是通过分子中原子的量子理论 (QTAIM) 分析确定的。探索了复杂的构象弛豫途径和相应的相互转换障碍,以使检测到的构象异构体的实验相对丰度合理化。分配了三种构象异构体的旋转光谱,包括天然丰度中最稳定构象异构体的四种 13C 同位素体和一种 18O 同位素体。其中一个检测到的构象异构体表现出在 –CH2OH 基团的非羟基氢原子和 –CF3 基团的氟原子之间形成的分子内氢键,这是通过分子中原子的量子理论 (QTAIM) 分析确定的。探索了复杂的构象弛豫途径和相应的相互转换障碍,以使检测到的构象异构体的实验相对丰度合理化。其中一个检测到的构象异构体表现出在 –CH2OH 基团的非羟基氢原子和 –CF3 基团的氟原子之间形成的分子内氢键,这是通过分子中原子的量子理论 (QTAIM) 分析确定的。探索了复杂的构象弛豫途径和相应的相互转换障碍,以使检测到的构象异构体的实验相对丰度合理化。其中一个检测到的构象异构体表现出在 –CH2OH 基团的非羟基氢原子和 –CF3 基团的氟原子之间形成的分子内氢键,这是通过分子中原子的量子理论 (QTAIM) 分析确定的。探索了复杂的构象弛豫途径和相应的相互转换障碍,以使检测到的构象异构体的实验相对丰度合理化。
更新日期:2020-10-01
中文翻译:

4,4,4-三氟-1-丁醇的构象景观和复杂的构象弛豫路径:旋转光谱和量子化学计算
摘要 含氟脂肪醇引起了相当大的兴趣,部分原因是它们被广泛用作肽和蛋白质的溶剂以及一些化学反应。在目前的工作中,使用啁啾脉冲傅里叶变换微波光谱仪结合脉冲分子膨胀研究了 4,4,4-三氟-1-丁醇的旋转光谱。4,4,4-三氟-1-丁醇中的长碳链和OH基团提供了显着的构象灵活性。使用基于紧束缚的半经验量子化学程序以及通过进行系统的手动构象搜索,总共确定了 27 种可能的几何形状。这些构象在 B3LYP-D3(BJ)/def2-TZVP、B3LYP-D3(BJ)/6–311++G(2d,p) 和 MP2/6–311++G(2d ,p) 理论水平。在 18 kJ mol-1 的能量窗口内获得了 14 个在光谱上可区分的构象异构体。分配了三种构象异构体的旋转光谱,包括天然丰度中最稳定构象异构体的四种 13C 同位素体和一种 18O 同位素体。其中一个检测到的构象异构体表现出在 –CH2OH 基团的非羟基氢原子和 –CF3 基团的氟原子之间形成的分子内氢键,这是通过分子中原子的量子理论 (QTAIM) 分析确定的。探索了复杂的构象弛豫途径和相应的相互转换障碍,以使检测到的构象异构体的实验相对丰度合理化。分配了三种构象异构体的旋转光谱,包括天然丰度中最稳定构象异构体的四种 13C 同位素体和一种 18O 同位素体。其中一个检测到的构象异构体表现出在 –CH2OH 基团的非羟基氢原子和 –CF3 基团的氟原子之间形成的分子内氢键,这是通过分子中原子的量子理论 (QTAIM) 分析确定的。探索了复杂的构象弛豫途径和相应的相互转换障碍,以使检测到的构象异构体的实验相对丰度合理化。分配了三种构象异构体的旋转光谱,包括天然丰度中最稳定构象异构体的四种 13C 同位素体和一种 18O 同位素体。其中一个检测到的构象异构体表现出在 –CH2OH 基团的非羟基氢原子和 –CF3 基团的氟原子之间形成的分子内氢键,这是通过分子中原子的量子理论 (QTAIM) 分析确定的。探索了复杂的构象弛豫途径和相应的相互转换障碍,以使检测到的构象异构体的实验相对丰度合理化。其中一个检测到的构象异构体表现出在 –CH2OH 基团的非羟基氢原子和 –CF3 基团的氟原子之间形成的分子内氢键,这是通过分子中原子的量子理论 (QTAIM) 分析确定的。探索了复杂的构象弛豫途径和相应的相互转换障碍,以使检测到的构象异构体的实验相对丰度合理化。其中一个检测到的构象异构体表现出在 –CH2OH 基团的非羟基氢原子和 –CF3 基团的氟原子之间形成的分子内氢键,这是通过分子中原子的量子理论 (QTAIM) 分析确定的。探索了复杂的构象弛豫途径和相应的相互转换障碍,以使检测到的构象异构体的实验相对丰度合理化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号