当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium-Catalyzed Asymmetric N-Acyl Nitrene Transfer Reaction: Imidation of Sulfide.
Organic Letters ( IF 4.9 ) Pub Date : 2020-05-01 , DOI: 10.1021/acs.orglett.0c01373 Masaki Yoshitake 1 , Hiroki Hayashi 2 , Tatsuya Uchida 2, 3
Organic Letters ( IF 4.9 ) Pub Date : 2020-05-01 , DOI: 10.1021/acs.orglett.0c01373 Masaki Yoshitake 1 , Hiroki Hayashi 2 , Tatsuya Uchida 2, 3
Affiliation
|
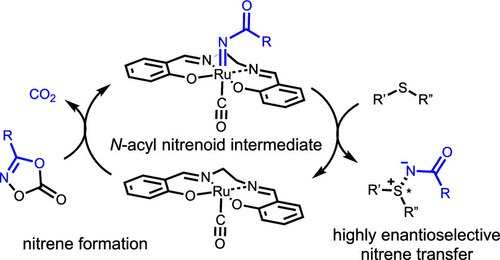
The asymmetric nitrene transfer reaction is a useful and strong tool for the construction of nitrogen functional groups such as N-sulfonyl amide and carbamic ester in a highly enantioselective manner. On the other hand, there is a substantial limitation in this filed: the transfer of N-acyl amide via the corresponding nitrene intermediates is still difficult because N-acyl nitrenes undergo undesired nitrene dimerization or Curtius rearrangement. Herein, we achieved highly enantioselective imidation of sulfides via catalytic N-acyl nitrene transfer with (OC)ruthenium-salen complex 2b as the catalyst and 3-substituted 1,4,2-dioxazol-5-ones 1 as the nitrene source. Complex 2b can decompose dioxazolones 1 to the desired N-acyl nitrene intermediates without any activation via heating or UV irradiation, or transfer generating nitrene intermediates to the sulfur atom of sulfides with good to excellent enantioselectivities (≤98% ee) without diazene and isocyanate contamination.
中文翻译:

钌催化的不对称N-酰基丁二烯转移反应:酰亚胺化的硫化物。
不对称腈转移反应是用于以高度对映选择性的方式构建氮官能团(例如N-磺酰基酰胺和氨基甲酸酯)的有用且强大的工具。另一方面,该领域存在很大的局限性:通过相应的腈中间体进行N-酰基酰胺的转移仍然是困难的,因为N-酰基腈经过不希望的腈二聚或Curtius重排。在本文中,我们通过催化的N-酰基腈转移反应,以(OC)钌-沙仑络合物2b为催化剂,3-取代的1,4,2-二恶唑-5-酮1为腈源,实现了硫化物的高度对映选择性酰亚胺化。配合物2b可以将二恶唑酮1分解为所需的N-酰基腈中间体,而无需通过加热或UV辐射进行任何活化,
更新日期:2020-05-01
中文翻译:

钌催化的不对称N-酰基丁二烯转移反应:酰亚胺化的硫化物。
不对称腈转移反应是用于以高度对映选择性的方式构建氮官能团(例如N-磺酰基酰胺和氨基甲酸酯)的有用且强大的工具。另一方面,该领域存在很大的局限性:通过相应的腈中间体进行N-酰基酰胺的转移仍然是困难的,因为N-酰基腈经过不希望的腈二聚或Curtius重排。在本文中,我们通过催化的N-酰基腈转移反应,以(OC)钌-沙仑络合物2b为催化剂,3-取代的1,4,2-二恶唑-5-酮1为腈源,实现了硫化物的高度对映选择性酰亚胺化。配合物2b可以将二恶唑酮1分解为所需的N-酰基腈中间体,而无需通过加热或UV辐射进行任何活化,































 京公网安备 11010802027423号
京公网安备 11010802027423号