当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tandem Phospha‐Michael Addition/N‐Acylation/ Intramolecular Wittig Reaction of aza‐o‐Quinone Methides: Approaches to 2,3‐Disubstituted Indoles
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-04-29 , DOI: 10.1002/adsc.202000343 Ting‐Bi Hua 1, 2 , Fei Chao 1 , Long Wang 1 , Chen‐Yang Yan 1 , Cong Xiao 3 , Qing‐Qing Yang 1, 2 , Wen‐Jing Xiao 4
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-04-29 , DOI: 10.1002/adsc.202000343 Ting‐Bi Hua 1, 2 , Fei Chao 1 , Long Wang 1 , Chen‐Yang Yan 1 , Cong Xiao 3 , Qing‐Qing Yang 1, 2 , Wen‐Jing Xiao 4
Affiliation
|
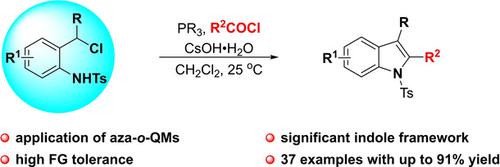
A tandem phospha‐Michael addition/N‐acylation/intramolecular Wittig reaction of in situ formed aza‐o‐QMs is disclosed. This approach features high functional group tolerance and provides a convenient and practical access to biologically significant indole derivatives (37 examples, up to 91% yield) under mild reaction conditions.
中文翻译:

氮杂邻苯二甲酰甲烷的串联磷酸-迈克尔加成反应/ N-酰化反应/分子内维蒂希反应:制备2,3-二取代吲哚的方法
一种串联磷迈克尔加成/ Ñ酰化分子内形成的原位Wittig反应氮杂/ ö -QMs中公开。该方法具有高度的官能团耐受性,在温和的反应条件下,可方便实用地获得生物学上重要的吲哚衍生物(37个实例,产率高达91%)。
更新日期:2020-04-29
中文翻译:

氮杂邻苯二甲酰甲烷的串联磷酸-迈克尔加成反应/ N-酰化反应/分子内维蒂希反应:制备2,3-二取代吲哚的方法
一种串联磷迈克尔加成/ Ñ酰化分子内形成的原位Wittig反应氮杂/ ö -QMs中公开。该方法具有高度的官能团耐受性,在温和的反应条件下,可方便实用地获得生物学上重要的吲哚衍生物(37个实例,产率高达91%)。































 京公网安备 11010802027423号
京公网安备 11010802027423号