当前位置:
X-MOL 学术
›
Diam. Relat. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of structural and electronic properties of N2O4 adsorbed on C20 fullerene
Diamond and Related Materials ( IF 4.3 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.diamond.2020.107836
Ferhat Demiray
Diamond and Related Materials ( IF 4.3 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.diamond.2020.107836
Ferhat Demiray
|
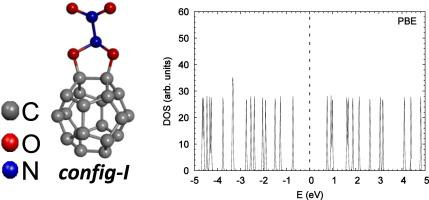
Abstract We report density functional theory calculations for structural and electronic properties of N2O4 adsorbed on C20 fullerene. At the beginning of the study, the isomers of N2O4 molecule were obtained and their structural properties were investigated. Our total energy calculations showed that the sym- N2O4 has lower energy than the other isomers. Therefore, it was selected as the most stable isomer of all. The structural properties of N2O4 isomers determined in this study were in agreement with previous studies. Various possible adsorption geometries were taken into consideration during the adsorption process of the N2O4 molecule onto the fullerene surface. The most stable four structures were obtained without any constraints by performing full structural optimizations. The adsorption energies of these optimized stable structures were calculated as −3.16, −3.35, −3.27, and −3.18 eV for LDA and −2.26, −2.61, −2.51, and −2.27 eV for PBE. These calculated values showed that the adsorption process can be evaluated as a chemisorption. GapHL values were calculated as 1.46, 1.41, 1.21, and 1.28 eV for LDA and 1.45, 0.34, 0.86, and 1.25 eV for PBE. The results obtained in this study regarding the N2O4 adsorption onto the C20 surfaces can be expected to guide future experimental and theoretical studies related to new hybrid semi-conductor material.
中文翻译:

吸附在 C20 富勒烯上的 N2O4 的结构和电子特性研究
摘要 我们报告了吸附在 C20 富勒烯上的 N2O4 的结构和电子特性的密度泛函理论计算。在研究开始时,获得了N2O4分子的异构体,并研究了它们的结构性质。我们的总能量计算表明,sym-N2O4 的能量低于其他异构体。因此,它被选为最稳定的异构体。本研究中确定的 N2O4 异构体的结构特性与之前的研究一致。在 N2O4 分子吸附到富勒烯表面的过程中,考虑了各种可能的吸附几何形状。通过执行完整的结构优化,在没有任何约束的情况下获得了最稳定的四种结构。这些优化的稳定结构的吸附能计算为 -3.16、-3.35、-3.27 和 -3.18 eV(对于 LDA)和 -2.26、-2.61、-2.51 和 -2.27 eV(对于 PBE)。这些计算值表明吸附过程可以作为化学吸附进行评估。LDA 的 GapHL 值计算为 1.46、1.41、1.21 和 1.28 eV,PBE 的计算值为 1.45、0.34、0.86 和 1.25 eV。本研究中获得的关于 N2O4 吸附到 C20 表面的结果有望指导未来与新型混合半导体材料相关的实验和理论研究。PBE 为 0.86 和 1.25 eV。本研究中获得的关于 N2O4 吸附到 C20 表面的结果有望指导未来与新型混合半导体材料相关的实验和理论研究。PBE 为 0.86 和 1.25 eV。本研究中获得的关于 N2O4 吸附到 C20 表面的结果有望指导未来与新型混合半导体材料相关的实验和理论研究。
更新日期:2020-08-01
中文翻译:

吸附在 C20 富勒烯上的 N2O4 的结构和电子特性研究
摘要 我们报告了吸附在 C20 富勒烯上的 N2O4 的结构和电子特性的密度泛函理论计算。在研究开始时,获得了N2O4分子的异构体,并研究了它们的结构性质。我们的总能量计算表明,sym-N2O4 的能量低于其他异构体。因此,它被选为最稳定的异构体。本研究中确定的 N2O4 异构体的结构特性与之前的研究一致。在 N2O4 分子吸附到富勒烯表面的过程中,考虑了各种可能的吸附几何形状。通过执行完整的结构优化,在没有任何约束的情况下获得了最稳定的四种结构。这些优化的稳定结构的吸附能计算为 -3.16、-3.35、-3.27 和 -3.18 eV(对于 LDA)和 -2.26、-2.61、-2.51 和 -2.27 eV(对于 PBE)。这些计算值表明吸附过程可以作为化学吸附进行评估。LDA 的 GapHL 值计算为 1.46、1.41、1.21 和 1.28 eV,PBE 的计算值为 1.45、0.34、0.86 和 1.25 eV。本研究中获得的关于 N2O4 吸附到 C20 表面的结果有望指导未来与新型混合半导体材料相关的实验和理论研究。PBE 为 0.86 和 1.25 eV。本研究中获得的关于 N2O4 吸附到 C20 表面的结果有望指导未来与新型混合半导体材料相关的实验和理论研究。PBE 为 0.86 和 1.25 eV。本研究中获得的关于 N2O4 吸附到 C20 表面的结果有望指导未来与新型混合半导体材料相关的实验和理论研究。

































 京公网安备 11010802027423号
京公网安备 11010802027423号