当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Charge-Sensitive Cluster-π Interactions Cause Altered Reactivity of Aln±,0 Clusters with Benzene: Enhanced Stability of Al13+Bz.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-05-12 , DOI: 10.1021/acs.jpca.0c02350 Mengzhou Yang 1, 2 , Hanyu Zhang 1, 2 , Yuhan Jia 1, 2 , Baoqi Yin 1, 2 , Zhixun Luo 1, 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-05-12 , DOI: 10.1021/acs.jpca.0c02350 Mengzhou Yang 1, 2 , Hanyu Zhang 1, 2 , Yuhan Jia 1, 2 , Baoqi Yin 1, 2 , Zhixun Luo 1, 2
Affiliation
|
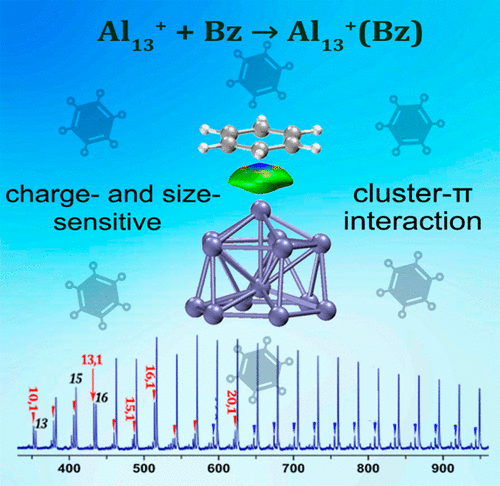
Utilizing the homemade reflection time-of-flight mass spectrometer (Re-TOFMS), here we report a comprehensive study of the reactivity of aluminum clusters Aln±,0 with molecular benzene in the gas-phase flow tube reactor. During the reactions with benzene, Aln+ clusters were found to be relatively more reactive than Aln0/-, and interestingly, the Al13+ cluster exhibited more reaction product than its neighboring Aln+ clusters. With an emphasis on Al13±,0 clusters, we have performed an in-depth study utilizing DFT calculations to unravel the diverse reactivity of aluminum clusters with benzene. It is revealed that the Al13+Bz cluster has a short Al-C distance and high binding energy, as well as an enlarged HOMO-LUMO gap in comparison with that of Al13+. This contrasts with Al130/- and Al15+, of which the HOMO-LUMO gaps are reduced when the cluster binds with a benzene molecule. Further, the cluster-π interactions between aluminum clusters and benzene are fully demonstrated via topological analysis, natural bonding orbital (NBO) analysis, and noncovalent interaction plots based on independent gradient model (IGM). The unique gyro-like structure of Al13+ and cluster-π interaction induce uneven redistribution of charges on the 13- atoms of Al13+, enabling a tight Al-C bond with strong electrostatic attraction and orbital interactions, which largely differs from the weak orbital overlap and electrostatic repulsion between benzene molecule and Al130/- clusters.
中文翻译:

电荷敏感的团簇π相互作用会导致Aln±,0团簇与苯的反应性发生改变:Al13 + Bz的稳定性增强。
本文利用自制的反射飞行时间质谱仪(Re-TOFMS),对气相流管反应器中铝簇Aln±,0与分子苯的反应性进行了全面研究。在与苯的反应过程中,发现Aln +团簇比Aln0 /-具有更高的反应活性,有趣的是,Al13 +团簇比其相邻的Aln +团簇具有更多的反应产物。我们着重研究Al13±,0团簇,利用DFT计算进行了深入研究,以揭示铝团簇与苯的不同反应性。结果表明,与Al13 +相比,Al13 + Bz团簇具有较短的Al-C距离和较高的结合能,并且具有较大的HOMO-LUMO间隙。这与Al130 /-和Al15 +形成对比,当簇与苯分子结合时,其中的HOMO-LUMO间隙减小。此外,通过拓扑分析,自然键合轨道(NBO)分析和基于独立梯度模型(IGM)的非共价相互作用图,充分证明了铝簇与苯之间的簇-π相互作用。Al13 +的独特陀螺状结构和簇π相互作用引起Al13 +的13-原子上电荷的不均匀重新分布,从而实现具有强静电吸引和轨道相互作用的紧密Al-C键,这与弱的轨道重叠和苯分子与Al130 /-团簇之间的静电排斥。自然键合轨道(NBO)分析以及基于独立梯度模型(IGM)的非共价相互作用图。Al13 +的独特陀螺状结构和簇π相互作用引起Al13 +的13-原子上电荷的不均匀重新分布,从而实现具有强静电吸引和轨道相互作用的紧密Al-C键,这与弱的轨道重叠和苯分子与Al130 /-团簇之间的静电排斥。自然键合轨道(NBO)分析以及基于独立梯度模型(IGM)的非共价相互作用图。Al13 +的独特陀螺状结构和簇π相互作用引起Al13 +的13-原子上电荷的不均匀重新分布,从而实现具有强静电吸引和轨道相互作用的紧密Al-C键,这与弱的轨道重叠和苯分子与Al130 /-团簇之间的静电排斥。
更新日期:2020-04-30
中文翻译:

电荷敏感的团簇π相互作用会导致Aln±,0团簇与苯的反应性发生改变:Al13 + Bz的稳定性增强。
本文利用自制的反射飞行时间质谱仪(Re-TOFMS),对气相流管反应器中铝簇Aln±,0与分子苯的反应性进行了全面研究。在与苯的反应过程中,发现Aln +团簇比Aln0 /-具有更高的反应活性,有趣的是,Al13 +团簇比其相邻的Aln +团簇具有更多的反应产物。我们着重研究Al13±,0团簇,利用DFT计算进行了深入研究,以揭示铝团簇与苯的不同反应性。结果表明,与Al13 +相比,Al13 + Bz团簇具有较短的Al-C距离和较高的结合能,并且具有较大的HOMO-LUMO间隙。这与Al130 /-和Al15 +形成对比,当簇与苯分子结合时,其中的HOMO-LUMO间隙减小。此外,通过拓扑分析,自然键合轨道(NBO)分析和基于独立梯度模型(IGM)的非共价相互作用图,充分证明了铝簇与苯之间的簇-π相互作用。Al13 +的独特陀螺状结构和簇π相互作用引起Al13 +的13-原子上电荷的不均匀重新分布,从而实现具有强静电吸引和轨道相互作用的紧密Al-C键,这与弱的轨道重叠和苯分子与Al130 /-团簇之间的静电排斥。自然键合轨道(NBO)分析以及基于独立梯度模型(IGM)的非共价相互作用图。Al13 +的独特陀螺状结构和簇π相互作用引起Al13 +的13-原子上电荷的不均匀重新分布,从而实现具有强静电吸引和轨道相互作用的紧密Al-C键,这与弱的轨道重叠和苯分子与Al130 /-团簇之间的静电排斥。自然键合轨道(NBO)分析以及基于独立梯度模型(IGM)的非共价相互作用图。Al13 +的独特陀螺状结构和簇π相互作用引起Al13 +的13-原子上电荷的不均匀重新分布,从而实现具有强静电吸引和轨道相互作用的紧密Al-C键,这与弱的轨道重叠和苯分子与Al130 /-团簇之间的静电排斥。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号