当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering nano-ordered of Ni nanoparticles on KIT-6 for enhanced catalytic hydrogenation of nitrobenzene
Applied Surface Science ( IF 6.3 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.apsusc.2020.146382 Xueke Zhou , Haitao Zhao , Shaojun Liu , Dong Ye , Ruiyang Qu , Chenghang Zheng , Xiang Gao
Applied Surface Science ( IF 6.3 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.apsusc.2020.146382 Xueke Zhou , Haitao Zhao , Shaojun Liu , Dong Ye , Ruiyang Qu , Chenghang Zheng , Xiang Gao
|
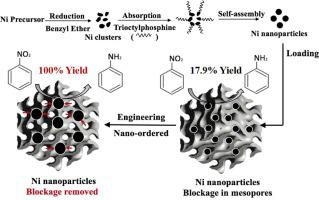
Abstract Catalytic hydrogenation of nitrobenzene by Ni nanoparticles on supports with high surface area is an economical and green route to produce aniline. To overcome the challenge of Ni nanoparticles blockage in the mesopores of KIT-6, a series of Ni-based KIT-6 catalysts were synthesized by designed engineering approaches. It was found that the engineering approaches were able to tune the nano-ordered of Ni nanoparticles and then remove the blockage on KIT-6, which significantly influence catalytic properties for nitrobenzene hydrogenation. The proposed engineering nano-ordered Ni nanoparticles blockage removal (ENBR) mechanism was systematically characterized. The characterization results confirmed that Ni dispersion, valence and metal surface area were adjusted by the ENBR process. The Ni particles located in the pore were not agglomerated, while the Ni particles located on the surface re-ordered to form larger particles and expose Ni particles blocked in the KIT-6 pores, which is the key factor for the remarkable enhancement of catalytic activity. Among all catalysts, with a specific surface area of 521.4 m2/g and Ni metal surface of 88.2 m2/g, Ni/KIT-6cal+red exhibited the best catalytic activity. This work was promising for the development of supported Ni catalysts to improve catalytic performance by engineering approaches.
中文翻译:

在 KIT-6 上设计纳米有序的 Ni 纳米颗粒以增强硝基苯的催化加氢
摘要 Ni纳米颗粒在高表面积载体上催化加氢硝基苯是一种经济、绿色的生产苯胺的途径。为了克服 KIT-6 介孔中 Ni 纳米颗粒堵塞的挑战,通过设计的工程方法合成了一系列 Ni 基 KIT-6 催化剂。结果发现,工程方法能够调整 Ni 纳米颗粒的纳米有序度,然后去除 KIT-6 上的堵塞,这显着影响硝基苯加氢的催化性能。系统地表征了所提出的工程纳米有序镍纳米粒子堵塞去除(ENBR)机制。表征结果证实了通过 ENBR 工艺调整了 Ni 的分散性、价态和金属表面积。位于孔隙中的 Ni 颗粒没有团聚,而位于表面的 Ni 颗粒重新排列形成更大的颗粒并暴露出封闭在 KIT-6 孔中的 Ni 颗粒,这是催化活性显着提高的关键因素。在所有催化剂中,Ni/KIT-6cal+red 的比表面积为 521.4 m2/g,Ni 金属表面积为 88.2 m2/g,表现出最好的催化活性。这项工作有望开发负载型 Ni 催化剂,以通过工程方法提高催化性能。
更新日期:2020-09-01
中文翻译:

在 KIT-6 上设计纳米有序的 Ni 纳米颗粒以增强硝基苯的催化加氢
摘要 Ni纳米颗粒在高表面积载体上催化加氢硝基苯是一种经济、绿色的生产苯胺的途径。为了克服 KIT-6 介孔中 Ni 纳米颗粒堵塞的挑战,通过设计的工程方法合成了一系列 Ni 基 KIT-6 催化剂。结果发现,工程方法能够调整 Ni 纳米颗粒的纳米有序度,然后去除 KIT-6 上的堵塞,这显着影响硝基苯加氢的催化性能。系统地表征了所提出的工程纳米有序镍纳米粒子堵塞去除(ENBR)机制。表征结果证实了通过 ENBR 工艺调整了 Ni 的分散性、价态和金属表面积。位于孔隙中的 Ni 颗粒没有团聚,而位于表面的 Ni 颗粒重新排列形成更大的颗粒并暴露出封闭在 KIT-6 孔中的 Ni 颗粒,这是催化活性显着提高的关键因素。在所有催化剂中,Ni/KIT-6cal+red 的比表面积为 521.4 m2/g,Ni 金属表面积为 88.2 m2/g,表现出最好的催化活性。这项工作有望开发负载型 Ni 催化剂,以通过工程方法提高催化性能。













































 京公网安备 11010802027423号
京公网安备 11010802027423号