当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Uptake of Nanomedicines at Different Stages of Brain Cancer Using a Modular Nanocarrier Platform and Precision Bispecific Antibodies.
ACS Central Science ( IF 12.7 ) Pub Date : 2020-04-28 , DOI: 10.1021/acscentsci.9b01299
Zachary H Houston 1, 2, 3 , Jens Bunt 4 , Kok-Siong Chen 4, 5 , Simon Puttick 2, 6 , Christopher B Howard 1, 2, 3, 7, 8 , Nicholas L Fletcher 1, 2, 3 , Adrian V Fuchs 1, 2, 3 , Jiwei Cui 3, 9, 10 , Yi Ju 3, 9 , Gary Cowin 1 , Xin Song 1 , Andrew W Boyd 11, 12 , Stephen M Mahler 2, 8 , Linda J Richards 4, 13 , Frank Caruso 3, 9 , Kristofer J Thurecht 1, 2, 3, 7
ACS Central Science ( IF 12.7 ) Pub Date : 2020-04-28 , DOI: 10.1021/acscentsci.9b01299
Zachary H Houston 1, 2, 3 , Jens Bunt 4 , Kok-Siong Chen 4, 5 , Simon Puttick 2, 6 , Christopher B Howard 1, 2, 3, 7, 8 , Nicholas L Fletcher 1, 2, 3 , Adrian V Fuchs 1, 2, 3 , Jiwei Cui 3, 9, 10 , Yi Ju 3, 9 , Gary Cowin 1 , Xin Song 1 , Andrew W Boyd 11, 12 , Stephen M Mahler 2, 8 , Linda J Richards 4, 13 , Frank Caruso 3, 9 , Kristofer J Thurecht 1, 2, 3, 7
Affiliation
|
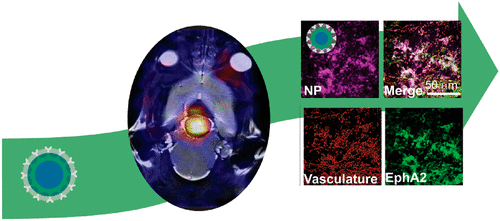
Increasing accumulation and retention of nanomedicines within tumor tissue is a significant challenge, particularly in the case of brain tumors where access to the tumor through the vasculature is restricted by the blood–brain barrier (BBB). This makes the application of nanomedicines in neuro-oncology often considered unfeasible, with efficacy limited to regions of significant disease progression and compromised BBB. However, little is understood about how the evolving tumor–brain physiology during disease progression affects the permeability and retention of designer nanomedicines. We report here the development of a modular nanomedicine platform that, when used in conjunction with a unique model of how tumorigenesis affects BBB integrity, allows investigation of how nanomaterial properties affect uptake and retention in brain tissue. By combining different in vivo longitudinal imaging techniques (including positron emission tomography and magnetic resonance imaging), we have evaluated the retention of nanomedicines with predefined physicochemical properties (size and surface functionality) and established a relationship between structure and tissue accumulation as a function of a new parameter that measures BBB leakiness; this offers significant advancements in our ability to relate tumor accumulation of nanomedicines to more physiologically relevant parameters. Our data show that accumulation of nanomedicines in brain tumor tissue is better correlated with the leakiness of the BBB than actual tumor volume. This was evaluated by establishing brain tumors using a spontaneous and endogenously derived glioblastoma model providing a unique opportunity to assess these parameters individually and compare the results across multiple mice. We also quantitatively demonstrate that smaller nanomedicines (20 nm) can indeed cross the BBB and accumulate in tumors at earlier stages of the disease than larger analogues, therefore opening the possibility of developing patient-specific nanoparticle treatment interventions in earlier stages of the disease. Importantly, these results provide a more predictive approach for designing efficacious personalized nanomedicines based on a particular patient’s condition.
中文翻译:

使用模块化纳米载体平台和精密双特异性抗体了解脑癌不同阶段纳米药物的吸收。
增加纳米药物在肿瘤组织内的积累和保留是一个重大挑战,特别是在脑肿瘤的情况下,通过脉管系统进入肿瘤受到血脑屏障(BBB)的限制。这使得纳米药物在神经肿瘤学中的应用通常被认为是不可行的,其功效仅限于疾病严重进展和血脑屏障受损的区域。然而,人们对疾病进展过程中不断变化的肿瘤脑生理学如何影响设计纳米药物的渗透性和保留性知之甚少。我们在这里报告模块化纳米医学平台的开发,当与肿瘤发生如何影响血脑屏障完整性的独特模型结合使用时,可以研究纳米材料特性如何影响脑组织的摄取和保留。通过结合不同的体内纵向成像技术(包括正电子发射断层扫描和磁共振成像),我们评估了具有预定物理化学性质(尺寸和表面功能)的纳米药物的保留,并建立了结构和组织积累之间的关系作为测量 BBB 泄漏的新参数;这极大地提高了我们将纳米药物的肿瘤积累与更多生理相关参数联系起来的能力。我们的数据表明,纳米药物在脑肿瘤组织中的积累与血脑屏障的渗漏性的相关性比实际肿瘤体积的相关性更好。 这是通过使用自发和内源性胶质母细胞瘤模型建立脑肿瘤来评估的,该模型提供了单独评估这些参数并比较多只小鼠的结果的独特机会。我们还定量地证明,与较大的类似物相比,较小的纳米药物(20 nm)确实可以穿过血脑屏障并在疾病的早期阶段在肿瘤中积累,因此为在疾病的早期阶段开发针对患者的特定纳米颗粒治疗干预措施提供了可能性。重要的是,这些结果为根据特定患者的病情设计有效的个性化纳米药物提供了更具预测性的方法。
更新日期:2020-04-28
中文翻译:

使用模块化纳米载体平台和精密双特异性抗体了解脑癌不同阶段纳米药物的吸收。
增加纳米药物在肿瘤组织内的积累和保留是一个重大挑战,特别是在脑肿瘤的情况下,通过脉管系统进入肿瘤受到血脑屏障(BBB)的限制。这使得纳米药物在神经肿瘤学中的应用通常被认为是不可行的,其功效仅限于疾病严重进展和血脑屏障受损的区域。然而,人们对疾病进展过程中不断变化的肿瘤脑生理学如何影响设计纳米药物的渗透性和保留性知之甚少。我们在这里报告模块化纳米医学平台的开发,当与肿瘤发生如何影响血脑屏障完整性的独特模型结合使用时,可以研究纳米材料特性如何影响脑组织的摄取和保留。通过结合不同的体内纵向成像技术(包括正电子发射断层扫描和磁共振成像),我们评估了具有预定物理化学性质(尺寸和表面功能)的纳米药物的保留,并建立了结构和组织积累之间的关系作为测量 BBB 泄漏的新参数;这极大地提高了我们将纳米药物的肿瘤积累与更多生理相关参数联系起来的能力。我们的数据表明,纳米药物在脑肿瘤组织中的积累与血脑屏障的渗漏性的相关性比实际肿瘤体积的相关性更好。 这是通过使用自发和内源性胶质母细胞瘤模型建立脑肿瘤来评估的,该模型提供了单独评估这些参数并比较多只小鼠的结果的独特机会。我们还定量地证明,与较大的类似物相比,较小的纳米药物(20 nm)确实可以穿过血脑屏障并在疾病的早期阶段在肿瘤中积累,因此为在疾病的早期阶段开发针对患者的特定纳米颗粒治疗干预措施提供了可能性。重要的是,这些结果为根据特定患者的病情设计有效的个性化纳米药物提供了更具预测性的方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号