当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen-Bonding Interactions in Hybrid Aqueous/Nonaqueous Electrolytes Enable Low-Cost and Long-Lifespan Sodium-Ion Storage.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-05-08 , DOI: 10.1021/acsami.0c03423
Rodney Chua 1 , Yi Cai 1 , Pei Qi Lim 1 , Sonal Kumar 1 , Rohit Satish 2 , William Manalastas 1 , Hao Ren 1 , Vivek Verma 1 , Shize Meng 1 , Samuel A Morris 1 , Pinit Kidkhunthod 3 , Jianming Bai 4 , Madhavi Srinivasan 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-05-08 , DOI: 10.1021/acsami.0c03423
Rodney Chua 1 , Yi Cai 1 , Pei Qi Lim 1 , Sonal Kumar 1 , Rohit Satish 2 , William Manalastas 1 , Hao Ren 1 , Vivek Verma 1 , Shize Meng 1 , Samuel A Morris 1 , Pinit Kidkhunthod 3 , Jianming Bai 4 , Madhavi Srinivasan 1
Affiliation
|
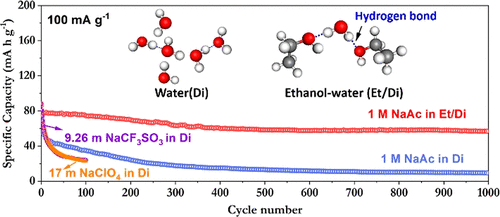
Although "water-in-salt" electrolytes have opened a new pathway to expand the electrochemical stability window of aqueous electrolytes, the electrode instability and irreversible proton co-insertion caused by aqueous media still hinder the practical application, even when using exotic fluorinated salts. In this study, an accessible hybrid electrolyte class based on common sodium salts is proposed, and crucially an ethanol-rich media is introduced to achieve highly stable Na-ion electrochemistry. Here, ethanol exerts a strong hydrogen-bonding effect on water, simultaneously expanding the electrochemical stability window of the hybridized electrolyte to 2.5 V, restricting degradation activities, reducing transition metal dissolution from the cathode material, and improving electrolyte-electrode wettability. The binary ethanol-water solvent enables the impressive cycling of sodium-ion batteries based on perchlorate, chloride, and acetate electrolyte salts. Notably, a Na0.44MnO2 electrode exhibits both high capacity (81 mAh g-1) and a remarkably long cycle life >1000 cycles at 100 mA g-1 (a capacity decay rate per cycle of 0.024%) in a 1 M sodium acetate system. The Na0.44MnO2/Zn full cells also show excellent cycling stability and rate capability in a wide temperature range. The gained understanding of the hydrogen-bonding interactions in the hybridized electrolyte can provide new battery chemistry guidelines in designing promising candidates for developing low-cost and long-lifespan batteries based on other (Li+, K+, Zn2+, Mg2+, and Al3+) systems.
中文翻译:

混合水/非水电解质中的氢键相互作用可实现低成本和长寿命的钠离子存储。
尽管“盐包水”电解质为扩大水性电解质的电化学稳定性窗口开辟了一条新途径,但即使使用异质氟化盐,由水性介质引起的电极不稳定性和不可逆质子共插入仍然阻碍了实际应用。在这项研究中,提出了一种基于普通钠盐的可及的混合电解质类别,并且至关重要的是,引入了富含乙醇的介质以实现高度稳定的Na离子电化学。在此,乙醇对水具有很强的氢键作用,同时将杂化电解质的电化学稳定性窗口扩大到2.5 V,限制了降解活性,减少了过渡金属从正极材料中的溶解,并改善了电解质电极的润湿性。二元乙醇-水溶剂可实现基于高氯酸盐,氯化物和乙酸盐电解质盐的钠离子电池令人印象深刻的循环。值得注意的是,在1 M乙酸钠中,Na0.44MnO2电极在100 mA g-1时表现出高容量(81 mAh g-1)和极长的循环寿命(> 1000次循环)(每循环的容量衰减率为0.024%)系统。Na0.44MnO2 / Zn满电池在宽温度范围内也显示出出色的循环稳定性和倍率性能。对杂化电解质中氢键相互作用的了解可以为设计基于其他(Li +,K +,Zn2 +,Mg2 +和Al3 +)系统的低成本和长寿命电池的有前途的候选设计提供新的电池化学指南。和醋酸盐电解质盐。值得注意的是,在1 M乙酸钠中,Na0.44MnO2电极在100 mA g-1时表现出高容量(81 mAh g-1)和极长的循环寿命(> 1000次循环)(每循环的容量衰减率为0.024%)系统。Na0.44MnO2 / Zn满电池在宽温度范围内也显示出出色的循环稳定性和倍率性能。对杂化电解质中氢键相互作用的了解可以为设计基于其他(Li +,K +,Zn2 +,Mg2 +和Al3 +)系统的低成本和长寿命电池的有前途的候选设计提供新的电池化学指南。和醋酸盐电解质盐。值得注意的是,在1 M乙酸钠中,Na0.44MnO2电极在100 mA g-1时表现出高容量(81 mAh g-1)和极长的循环寿命(> 1000次循环)(每循环的容量衰减率为0.024%)系统。Na0.44MnO2 / Zn充满电池在宽温度范围内也显示出出色的循环稳定性和速率能力。对杂化电解质中氢键相互作用的了解可以为设计基于其他(Li +,K +,Zn2 +,Mg2 +和Al3 +)系统的低成本和长寿命电池的有前途的候选设计提供新的电池化学指南。在1 M醋酸钠系统中加入024%)。Na0.44MnO2 / Zn满电池在宽温度范围内也显示出出色的循环稳定性和倍率性能。对杂化电解质中氢键相互作用的了解可以为设计基于其他(Li +,K +,Zn2 +,Mg2 +和Al3 +)系统的低成本和长寿命电池的有前途的候选设计提供新的电池化学指南。在1 M醋酸钠系统中加入024%)。Na0.44MnO2 / Zn满电池在宽温度范围内也显示出出色的循环稳定性和倍率性能。对杂化电解质中氢键相互作用的了解可以为设计基于其他(Li +,K +,Zn2 +,Mg2 +和Al3 +)系统的低成本和长寿命电池的有前途的候选设计提供新的电池化学指南。
更新日期:2020-04-28
中文翻译:

混合水/非水电解质中的氢键相互作用可实现低成本和长寿命的钠离子存储。
尽管“盐包水”电解质为扩大水性电解质的电化学稳定性窗口开辟了一条新途径,但即使使用异质氟化盐,由水性介质引起的电极不稳定性和不可逆质子共插入仍然阻碍了实际应用。在这项研究中,提出了一种基于普通钠盐的可及的混合电解质类别,并且至关重要的是,引入了富含乙醇的介质以实现高度稳定的Na离子电化学。在此,乙醇对水具有很强的氢键作用,同时将杂化电解质的电化学稳定性窗口扩大到2.5 V,限制了降解活性,减少了过渡金属从正极材料中的溶解,并改善了电解质电极的润湿性。二元乙醇-水溶剂可实现基于高氯酸盐,氯化物和乙酸盐电解质盐的钠离子电池令人印象深刻的循环。值得注意的是,在1 M乙酸钠中,Na0.44MnO2电极在100 mA g-1时表现出高容量(81 mAh g-1)和极长的循环寿命(> 1000次循环)(每循环的容量衰减率为0.024%)系统。Na0.44MnO2 / Zn满电池在宽温度范围内也显示出出色的循环稳定性和倍率性能。对杂化电解质中氢键相互作用的了解可以为设计基于其他(Li +,K +,Zn2 +,Mg2 +和Al3 +)系统的低成本和长寿命电池的有前途的候选设计提供新的电池化学指南。和醋酸盐电解质盐。值得注意的是,在1 M乙酸钠中,Na0.44MnO2电极在100 mA g-1时表现出高容量(81 mAh g-1)和极长的循环寿命(> 1000次循环)(每循环的容量衰减率为0.024%)系统。Na0.44MnO2 / Zn满电池在宽温度范围内也显示出出色的循环稳定性和倍率性能。对杂化电解质中氢键相互作用的了解可以为设计基于其他(Li +,K +,Zn2 +,Mg2 +和Al3 +)系统的低成本和长寿命电池的有前途的候选设计提供新的电池化学指南。和醋酸盐电解质盐。值得注意的是,在1 M乙酸钠中,Na0.44MnO2电极在100 mA g-1时表现出高容量(81 mAh g-1)和极长的循环寿命(> 1000次循环)(每循环的容量衰减率为0.024%)系统。Na0.44MnO2 / Zn充满电池在宽温度范围内也显示出出色的循环稳定性和速率能力。对杂化电解质中氢键相互作用的了解可以为设计基于其他(Li +,K +,Zn2 +,Mg2 +和Al3 +)系统的低成本和长寿命电池的有前途的候选设计提供新的电池化学指南。在1 M醋酸钠系统中加入024%)。Na0.44MnO2 / Zn满电池在宽温度范围内也显示出出色的循环稳定性和倍率性能。对杂化电解质中氢键相互作用的了解可以为设计基于其他(Li +,K +,Zn2 +,Mg2 +和Al3 +)系统的低成本和长寿命电池的有前途的候选设计提供新的电池化学指南。在1 M醋酸钠系统中加入024%)。Na0.44MnO2 / Zn满电池在宽温度范围内也显示出出色的循环稳定性和倍率性能。对杂化电解质中氢键相互作用的了解可以为设计基于其他(Li +,K +,Zn2 +,Mg2 +和Al3 +)系统的低成本和长寿命电池的有前途的候选设计提供新的电池化学指南。



































 京公网安备 11010802027423号
京公网安备 11010802027423号