当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lithium bis(oxalate)borate additive in the electrolyte to improve Li-rich layered oxide cathode materials
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2020-04-28 , DOI: 10.1039/d0qm00094a Zi Xiao 1, 2, 3, 4, 5 , Jiuding Liu 1, 2, 3, 4, 5 , Guilan Fan 1, 2, 3, 4, 5 , Meng Yu 1, 2, 3, 4, 5 , Junxiang Liu 1, 2, 3, 4, 5 , Xinglong Gou 6, 7, 8, 9, 10 , Mingjian Yuan 1, 2, 3, 4, 5 , Fangyi Cheng 1, 2, 3, 4, 5
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2020-04-28 , DOI: 10.1039/d0qm00094a Zi Xiao 1, 2, 3, 4, 5 , Jiuding Liu 1, 2, 3, 4, 5 , Guilan Fan 1, 2, 3, 4, 5 , Meng Yu 1, 2, 3, 4, 5 , Junxiang Liu 1, 2, 3, 4, 5 , Xinglong Gou 6, 7, 8, 9, 10 , Mingjian Yuan 1, 2, 3, 4, 5 , Fangyi Cheng 1, 2, 3, 4, 5
Affiliation
|
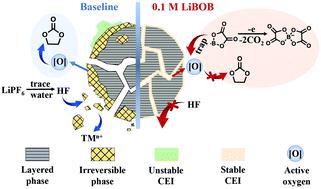
Lithium-rich layered oxides (LLO), as the most attractive cathode materials for high-energy lithium-ion batteries (LIBs), are plagued by poor cyclability due to structural and electrode/electrolyte interface instability. Herein, we report the synthesis of a LLO and its performance enhancement by using boron-containing electrolyte additives. In a formulated 1.0 M LiPF6 ethylene carbonate/ethyl methyl carbonate electrolyte with 0.1 M lithium bis(oxalato)borate (LiBOB), the battery assembled with Li1.2Mn0.54Co0.13Ni0.13O2 microspheres presents a stable specific capacity of 202 mA h g−1 at 0.5C and a remarkable capacity retention of 96.4% after 100 cycles, significantly outperforming the cathode in the baseline electrolyte without LiBOB. The combination of voltammetry, impedance, microscopy, and spectroscopic analysis and density functional theory (DFT) calculations corroborates the beneficial effect of LiBOB in stabilizing the LLO/electrolyte interface. Reactions between LiBOB and activated oxygen radicals result in the formation of a dense cathode electrolyte interface (CEI) film (∼15 nm) containing oxalate, lithium fluoride and alkyl borate species, which contributes to suppression of the capacity/voltage decay of the LLO. These results would provide insight in understanding the effect of boron-containing electrolyte additives in upgrading high-capacity Li-rich cathode materials.
中文翻译:

电解液中的双(草酸)硼酸锂添加剂可改善富锂的分层氧化物阴极材料
富锂层状氧化物(LLO)作为高能锂离子电池(LIB)最具吸引力的阴极材料,由于结构和电极/电解质界面的不稳定性而导致循环性能差,从而困扰着他们。在此,我们报道了通过使用含硼电解质添加剂的LLO的合成及其性能增强。在带有0.1 M双(草酸)硼酸锂(LiBOB)的配制的1.0 M LiPF 6碳酸亚乙酯/碳酸乙基甲酯电解质中,由Li 1.2 Mn 0.54 Co 0.13 Ni 0.13 O 2微球组装而成的电池具有202 mA的稳定比容量。汞-1在0.5C下,在100个循环后,其显着的容量保持率达到96.4%,在不使用LiBOB的基线电解质中,其性能明显优于阴极。伏安法,阻抗法,显微镜法,光谱分析法和密度泛函理论(DFT)计算的结合,证实了LiBOB在稳定LLO /电解质界面方面的有益作用。LiBOB与活化氧自由基之间的反应导致形成了包含草酸根,氟化锂和烷基硼酸根物质的致密阴极电解质界面(CEI)膜(约15 nm),这有助于抑制LLO的容量/电压衰减。这些结果将为理解含硼电解质添加剂在升级高容量富锂阴极材料方面的作用提供见识。
更新日期:2020-04-28
中文翻译:

电解液中的双(草酸)硼酸锂添加剂可改善富锂的分层氧化物阴极材料
富锂层状氧化物(LLO)作为高能锂离子电池(LIB)最具吸引力的阴极材料,由于结构和电极/电解质界面的不稳定性而导致循环性能差,从而困扰着他们。在此,我们报道了通过使用含硼电解质添加剂的LLO的合成及其性能增强。在带有0.1 M双(草酸)硼酸锂(LiBOB)的配制的1.0 M LiPF 6碳酸亚乙酯/碳酸乙基甲酯电解质中,由Li 1.2 Mn 0.54 Co 0.13 Ni 0.13 O 2微球组装而成的电池具有202 mA的稳定比容量。汞-1在0.5C下,在100个循环后,其显着的容量保持率达到96.4%,在不使用LiBOB的基线电解质中,其性能明显优于阴极。伏安法,阻抗法,显微镜法,光谱分析法和密度泛函理论(DFT)计算的结合,证实了LiBOB在稳定LLO /电解质界面方面的有益作用。LiBOB与活化氧自由基之间的反应导致形成了包含草酸根,氟化锂和烷基硼酸根物质的致密阴极电解质界面(CEI)膜(约15 nm),这有助于抑制LLO的容量/电压衰减。这些结果将为理解含硼电解质添加剂在升级高容量富锂阴极材料方面的作用提供见识。































 京公网安备 11010802027423号
京公网安备 11010802027423号