Neuron ( IF 14.7 ) Pub Date : 2020-04-27 , DOI: 10.1016/j.neuron.2020.03.033 Christopher L Cunningham 1 , Xufeng Qiu 1 , Zizhen Wu 1 , Bo Zhao 2 , Guihong Peng 1 , Ye-Hyun Kim 3 , Amanda Lauer 3 , Ulrich Müller 1
|
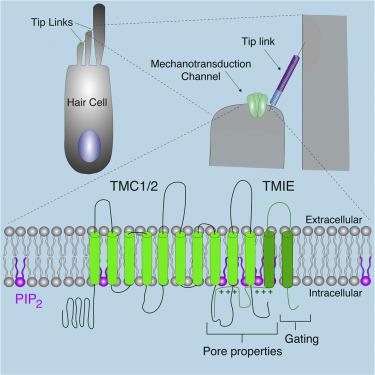
TMC1 and TMC2 (TMC1/2) have been proposed to form the pore of the mechanotransduction channel of cochlear hair cells. Here, we show that TMC1/2 cannot form mechanotransduction channels in cochlear hair cells without TMIE. TMIE binds to TMC1/2, and a TMIE mutation that perturbs TMC1/2 binding abolishes mechanotransduction. N-terminal TMIE deletions affect the response of the mechanotransduction channel to mechanical force. Similar to mechanically gated TREK channels, the C-terminal cytoplasmic TMIE domain contains charged amino acids that mediate binding to phospholipids, including PIP2. TMIE point mutations in the C terminus that are linked to deafness disrupt phospholipid binding, sensitize the channel to PIP2 depletion from hair cells, and alter the channel’s unitary conductance and ion selectivity. We conclude that TMIE is a subunit of the cochlear mechanotransduction channel and that channel function is regulated by a phospholipid-sensing domain in TMIE with similarity to those in other mechanically gated ion channels.
中文翻译:

TMIE 定义了哺乳动物耳蜗毛细胞机械传导通道的孔和门控特性。
TMC1 和 TMC2 (TMC1/2) 被提议形成耳蜗毛细胞的机械传导通道的孔。在这里,我们表明,如果没有 TMIE,TMC1/2 就无法在耳蜗毛细胞中形成机械转导通道。 TMIE 与 TMC1/2 结合,扰乱 TMC1/2 结合的 TMIE 突变会消除机械转导。 N 端 TMIE 缺失影响机械传导通道对机械力的响应。与机械门控 TREK 通道类似,C 端胞质 TMIE 结构域包含介导与磷脂(包括 PIP 2 )结合的带电氨基酸。与耳聋相关的 C 末端 TMIE 点突变会破坏磷脂结合,使通道对毛细胞 PIP 2消耗敏感,并改变通道的单一电导和离子选择性。我们得出结论,TMIE 是耳蜗机械转导通道的一个亚基,通道功能由 TMIE 中的磷脂感应结构域调节,与其他机械门控离子通道中的磷脂感应结构域相似。






























 京公网安备 11010802027423号
京公网安备 11010802027423号