当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Polysulfide Conversion and Physiochemical Confinement for Lithium–Sulfur Batteries
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-04-27 , DOI: 10.1002/aenm.201904010 Zixu Sun 1 , Sudarshan Vijay 2 , Hendrik H. Heenen 2 , Alex Yong Sheng Eng 3 , Wenguang Tu 1 , Yunxing Zhao 4 , See Wee Koh 1 , Pingqi Gao 5 , Zhi Wei Seh 3 , Karen Chan 2 , Hong Li 1, 6, 7
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-04-27 , DOI: 10.1002/aenm.201904010 Zixu Sun 1 , Sudarshan Vijay 2 , Hendrik H. Heenen 2 , Alex Yong Sheng Eng 3 , Wenguang Tu 1 , Yunxing Zhao 4 , See Wee Koh 1 , Pingqi Gao 5 , Zhi Wei Seh 3 , Karen Chan 2 , Hong Li 1, 6, 7
Affiliation
|
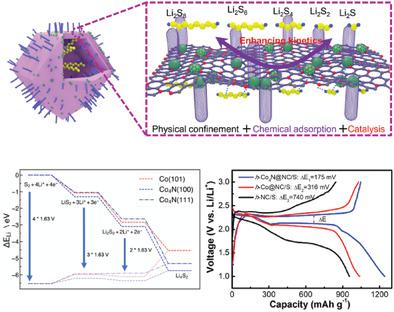
The lithium–sulfur (Li–S) battery is widely regarded as a promising energy storage device due to its low price and the high earth‐abundance of the materials employed. However, the shuttle effect of lithium polysulfides (LiPSs) and sluggish redox conversion result in inefficient sulfur utilization, low power density, and rapid electrode deterioration. Herein, these challenges are addressed with two strategies 1) increasing LiPS conversion kinetics through catalysis, and 2) alleviating the shuttle effect by enhanced trapping and adsorption of LiPSs. These improvements are achieved by constructing double‐shelled hollow nanocages decorated with a cobalt nitride catalyst. The N‐doped hollow inner carbon shell not only serves as a physiochemical absorber for LiPSs, but also improves the electrical conductivity of the electrode; significantly suppressing shuttle effect. Cobalt nitride (Co4N) nanoparticles, embedded in nitrogen‐doped carbon in the outer shell, catalyze the conversion of LiPSs, leading to decreased polarization and fast kinetics during cycling. Theoretical study of the Li intercalation energetics confirms the improved catalytic activity of the Co4N compared to metallic Co catalyst. Altogether, the electrode shows large reversible capacity (1242 mAh g−1 at 0.1 C), robust stability (capacity retention of 658 mAh g−1 at 5 C after 400 cycles), and superior cycling stability at high sulfur loading (4.5 mg cm−2).
中文翻译:

锂硫电池的催化多硫化物转化和物理化学限制
锂硫(Li–S)电池因其价格低廉和所用材料的高地球丰度而被广泛视为有前途的储能设备。但是,多硫化锂(LiPSs)的穿梭效应和缓慢的氧化还原转化会导致硫利用效率低,功率密度低和电极快速变质。本文中,这些挑战通过两种策略解决:1)通过催化提高LiPS转化动力学,以及2)通过增强LiPS的捕获和吸附来减轻穿梭效应。通过构建装饰有氮化钴催化剂的双壳空心纳米笼,可以实现这些改进。N掺杂空心碳壳不仅可以用作LiPS的物理化学吸收剂,还可以改善电极的导电性。显着抑制穿梭效果。氮化钴(Co4 N)纳米粒子嵌入外壳的氮掺杂碳中,催化LiPS的转化,导致极化降低和循环过程中的快速动力学。Li嵌入能量学的理论研究证实,与金属Co催化剂相比,Co 4 N的催化活性有所提高。总之,电极显示出大的可逆容量(0.1 C时为1242 mAh g -1),稳定的稳定性(400次循环后在5 C时的容量保持为658 mAh g -1)和在高硫负荷(4.5 mg cm)下的优异循环稳定性-2)。
更新日期:2020-04-27
中文翻译:

锂硫电池的催化多硫化物转化和物理化学限制
锂硫(Li–S)电池因其价格低廉和所用材料的高地球丰度而被广泛视为有前途的储能设备。但是,多硫化锂(LiPSs)的穿梭效应和缓慢的氧化还原转化会导致硫利用效率低,功率密度低和电极快速变质。本文中,这些挑战通过两种策略解决:1)通过催化提高LiPS转化动力学,以及2)通过增强LiPS的捕获和吸附来减轻穿梭效应。通过构建装饰有氮化钴催化剂的双壳空心纳米笼,可以实现这些改进。N掺杂空心碳壳不仅可以用作LiPS的物理化学吸收剂,还可以改善电极的导电性。显着抑制穿梭效果。氮化钴(Co4 N)纳米粒子嵌入外壳的氮掺杂碳中,催化LiPS的转化,导致极化降低和循环过程中的快速动力学。Li嵌入能量学的理论研究证实,与金属Co催化剂相比,Co 4 N的催化活性有所提高。总之,电极显示出大的可逆容量(0.1 C时为1242 mAh g -1),稳定的稳定性(400次循环后在5 C时的容量保持为658 mAh g -1)和在高硫负荷(4.5 mg cm)下的优异循环稳定性-2)。































 京公网安备 11010802027423号
京公网安备 11010802027423号