当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atmospheric Chemistry of Volatile Methyl Siloxanes: Kinetics and Products of Oxidation by OH Radicals and Cl Atoms.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-05-04 , DOI: 10.1021/acs.est.0c01368
Mitchell W Alton 1 , Eleanor C Browne 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-05-04 , DOI: 10.1021/acs.est.0c01368
Mitchell W Alton 1 , Eleanor C Browne 1
Affiliation
|
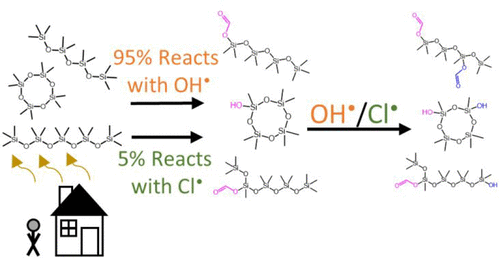
Volatile methyl siloxanes (VMS) are ubiquitous anthropogenic pollutants that have recently come under scrutiny for their potential toxicity and environmental persistence. In this work, we determined the rate constants for oxidation by OH radicals and Cl atoms at 297 ± 3 K and atmospheric pressure in Boulder, CO (∼860 mbar) of hexamethyldisiloxane (L2), octamethyltrisiloxane (L3), decamethyltetrasiloxane (L4), dodecamethylpentasiloxane (L5), hexamethylcyclotrisiloxane (D3), octamethylcyclotetrasiloxane (D4), and decamethylcyclopentasiloxane (D5). Measured rate constants with OH radicals were (1.20 ± 0.09) × 10-12, (1.7 ± 0.1) × 10-12, (2.5 ± 0.2) × 10-12, (3.4 ± 0.5) × 10-12, (0.86 ± 0.09) × 10-12, (1.3 ± 0.1) × 10-12, and (2.1 ± 0.1) × 10-12 cm3 molec-1 s-1, for L2, L3, L4, L5, D3, D4, and D5, respectively. The rate constants for reactions with Cl atoms with the same compounds were (1.44 ± 0.05) × 10-10, (1.85 ± 0.05) × 10-10, (2.2 ± 0.1) × 10-10, (2.9 ± 0.1) × 10-10, (0.56 ± 0.05) × 10-10, (1.16 ± 0.08) × 10-10, and (1.8 ± 0.1) × 10-10 cm3 molec-1 s-1, respectively. Substituent factors of F(-Si(CH3)2OR) and F(-SiCH3(OR)2) are proposed for use in AOPWIN, a common model for OH radical rate constant estimations. Cl atoms can remove percentage levels of VMS globally with potentially increased importance in urban areas.
中文翻译:

挥发性甲基硅氧烷的大气化学:OH自由基和Cl原子氧化的动力学和产物。
挥发性甲基硅氧烷(VMS)是普遍存在的人为污染物,最近因其潜在的毒性和环境持久性而受到严格审查。在这项工作中,我们确定了在297±3 K和大气压力下,在Boulder,CO(〜860 mbar)中,六甲基二硅氧烷(L2),八甲基三硅氧烷(L3),十甲基四硅氧烷(L4),十二甲基五硅氧烷(L5),六甲基环三硅氧烷(D3),八甲基环四硅氧烷(D4)和十甲基环五硅氧烷(D5)。使用OH自由基测得的速率常数为(1.20±0.09)×10-12,(1.7±0.1)×10-12,(2.5±0.2)×10-12,(3.4±0.5)×10-12,(0.86±对于L2,L3,L4,L5,D3,D4和D5,0.09)×10-12,(1.3±0.1)×10-12和(2.1±0.1)×10-12 cm3 molec-1 s-1 , 分别。与相同化合物的Cl原子反应的速率常数为(1.44±0.05)×10-10,(1.85±0.05)×10-10,(2.2±0.1)×10-10,(2.9±0.1)×10分别为-10,(0.56±0.05)×10-10,(1.16±0.08)×10-10和(1.8±0.1)×10-10 cm3 molec-1 s-1。提出了F(-Si(CH3)2OR)和F(-SiCH3(OR)2)的取代因子以用于AOPWIN,这是OH自由基速率常数估算的通用模型。Cl原子可以在全球范围内消除VMS的百分比水平,在城市地区的重要性可能会增加。OH自由基速率常数估算的通用模型。Cl原子可以在全球范围内消除VMS的百分比水平,在城市地区的重要性可能会增加。OH自由基速率常数估算的通用模型。Cl原子可以在全球范围内消除VMS的百分比水平,在城市地区的重要性可能会增加。
更新日期:2020-04-27
中文翻译:

挥发性甲基硅氧烷的大气化学:OH自由基和Cl原子氧化的动力学和产物。
挥发性甲基硅氧烷(VMS)是普遍存在的人为污染物,最近因其潜在的毒性和环境持久性而受到严格审查。在这项工作中,我们确定了在297±3 K和大气压力下,在Boulder,CO(〜860 mbar)中,六甲基二硅氧烷(L2),八甲基三硅氧烷(L3),十甲基四硅氧烷(L4),十二甲基五硅氧烷(L5),六甲基环三硅氧烷(D3),八甲基环四硅氧烷(D4)和十甲基环五硅氧烷(D5)。使用OH自由基测得的速率常数为(1.20±0.09)×10-12,(1.7±0.1)×10-12,(2.5±0.2)×10-12,(3.4±0.5)×10-12,(0.86±对于L2,L3,L4,L5,D3,D4和D5,0.09)×10-12,(1.3±0.1)×10-12和(2.1±0.1)×10-12 cm3 molec-1 s-1 , 分别。与相同化合物的Cl原子反应的速率常数为(1.44±0.05)×10-10,(1.85±0.05)×10-10,(2.2±0.1)×10-10,(2.9±0.1)×10分别为-10,(0.56±0.05)×10-10,(1.16±0.08)×10-10和(1.8±0.1)×10-10 cm3 molec-1 s-1。提出了F(-Si(CH3)2OR)和F(-SiCH3(OR)2)的取代因子以用于AOPWIN,这是OH自由基速率常数估算的通用模型。Cl原子可以在全球范围内消除VMS的百分比水平,在城市地区的重要性可能会增加。OH自由基速率常数估算的通用模型。Cl原子可以在全球范围内消除VMS的百分比水平,在城市地区的重要性可能会增加。OH自由基速率常数估算的通用模型。Cl原子可以在全球范围内消除VMS的百分比水平,在城市地区的重要性可能会增加。

































 京公网安备 11010802027423号
京公网安备 11010802027423号