当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ-Generated Multivalent Aptamer Network for Efficient Capture and Sensitive Electrochemical Detection of Circulating Tumor Cells in Whole Blood.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-04-27 , DOI: 10.1021/acs.analchem.0c01195 Jianmei Yang 1 , Xiaolong Li 2 , Bingying Jiang 2 , Ruo Yuan 1 , Yun Xiang 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-04-27 , DOI: 10.1021/acs.analchem.0c01195 Jianmei Yang 1 , Xiaolong Li 2 , Bingying Jiang 2 , Ruo Yuan 1 , Yun Xiang 1
Affiliation
|
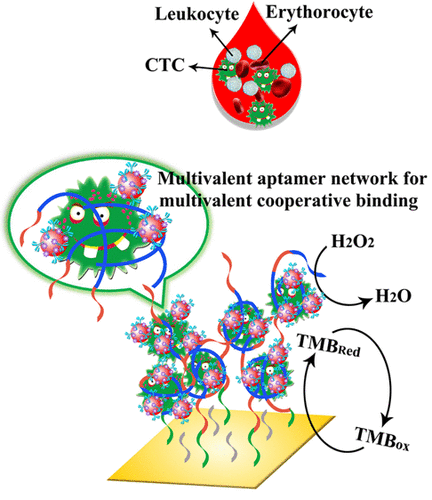
Monitoring circulating tumor cells (CTCs) in human blood can offer useful information for convenient metastasis diagnosis, prognosis, and treatment of cancers. However, it remains a substantial challenge to detect CTCs because of their particular scarcity in complex peripheral blood. Herein, we describe an in situ-generated multivalent aptamer network-modified electrode interface for efficiently capturing and sensitively detecting CTCs in whole blood by electrochemistry. Such an interface was fabricated via rolling circle amplification extension of the electrode-immobilized primer/circular DNA complexes for the yield of long ssDNA strands with many repeated aptamer segments, which could achieve efficient capture of rare CTCs in a multivalent cooperative manner. The antibody and horseradish peroxidase-functionalized gold nanoparticles further specifically associated with the surface-bound CTCs and generated electrocatalytically amplified current outputs for highly sensitive detection of CTCs with an attractive detection limit of five cells. Also, the multivalent aptamer network interface could successfully distinguish the target cells from other control cells and achieve CTC detection in whole blood, demonstrating its promising potential for monitoring different rare CTCs in human blood.
中文翻译:

原位生成的多价适体网络,用于全血中循环肿瘤细胞的高效捕获和灵敏的电化学检测。
监测人血中循环中的肿瘤细胞(CTC)可以为癌症的便捷转移诊断,预后和治疗提供有用的信息。但是,由于CTC在复杂的外周血中特别稀缺,因此检测CTC仍然是一个巨大的挑战。在这里,我们描述了一种原位产生的多价适体网络修饰的电极界面,用于通过电化学有效捕获和灵敏地检测全血中的四氯化碳。这样的接口是通过固定有电极的引物/环状DNA复合物的滚环扩增延伸,以产生具有许多重复的适体片段的长ssDNA链,从而可以以多价协作方式有效捕获稀有CTC。抗体和辣根过氧化物酶功能化的金纳米颗粒还与表面结合的四氯化碳进一步特异性结合,并产生了电催化放大的电流输出,用于高灵敏度检测四氯化碳,检测极限为五个细胞。而且,多价适体网络接口可以成功地将靶细胞与其他对照细胞区分开,并在全血中实现CTC检测,证明了其在监测人血中不同稀有CTC方面的潜力。
更新日期:2020-04-27
中文翻译:

原位生成的多价适体网络,用于全血中循环肿瘤细胞的高效捕获和灵敏的电化学检测。
监测人血中循环中的肿瘤细胞(CTC)可以为癌症的便捷转移诊断,预后和治疗提供有用的信息。但是,由于CTC在复杂的外周血中特别稀缺,因此检测CTC仍然是一个巨大的挑战。在这里,我们描述了一种原位产生的多价适体网络修饰的电极界面,用于通过电化学有效捕获和灵敏地检测全血中的四氯化碳。这样的接口是通过固定有电极的引物/环状DNA复合物的滚环扩增延伸,以产生具有许多重复的适体片段的长ssDNA链,从而可以以多价协作方式有效捕获稀有CTC。抗体和辣根过氧化物酶功能化的金纳米颗粒还与表面结合的四氯化碳进一步特异性结合,并产生了电催化放大的电流输出,用于高灵敏度检测四氯化碳,检测极限为五个细胞。而且,多价适体网络接口可以成功地将靶细胞与其他对照细胞区分开,并在全血中实现CTC检测,证明了其在监测人血中不同稀有CTC方面的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号