当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction between casein and rice glutelin: Binding mechanisms and molecular assembly behaviours
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.foodhyd.2020.105967
Chengxin He , Yu Hu , Ziwei Liu , Meng Wai Woo , Hua Xiong , Qiang Zhao
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.foodhyd.2020.105967
Chengxin He , Yu Hu , Ziwei Liu , Meng Wai Woo , Hua Xiong , Qiang Zhao
|
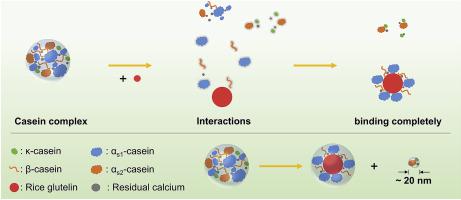
Abstract The present study investigated the interaction between acid casein and rice glutelin (RG) under neutral conditions. Large particle populations contain casein-RG complex which consisted of β-casein, αs1-casein, and RG was formed. The results of multispectral studies indicated that the peptide chain and secondary structure of casein did not change during the binding process. Besides, the binding behaviours seemed to be closely related to the surface hydrophobic region of RG. Thermodynamic parameter calculations showed that the main driving forces behind this structure formation were the hydrogen bonds and van der Waals forces. On the other hand, the addition of RG caused the generation of small particle populations (~20 nm) which mainly composed of κ-casein and αs2-casein, and such small particle populations maintained aggregation through residual calcium. Moreover, it is noting that these two particle populations can coexist stably, this finding will provide an avenue to produce, by design, casein nanoparticles.
中文翻译:

酪蛋白和大米谷蛋白的相互作用:结合机制和分子组装行为
摘要 本研究调查了酸性酪蛋白和大米谷蛋白(RG)在中性条件下的相互作用。大颗粒群含有酪蛋白-RG复合物,由β-酪蛋白、αs1-酪蛋白组成,形成RG。多光谱研究结果表明,酪蛋白的肽链和二级结构在结合过程中没有发生变化。此外,结合行为似乎与 RG 的表面疏水区密切相关。热力学参数计算表明,这种结构形成背后的主要驱动力是氢键和范德华力。另一方面,RG的加入导致产生主要由κ-酪蛋白和αs2-酪蛋白组成的小颗粒群(~20 nm),并且这种小颗粒群通过残留的钙保持聚集。此外,注意到这两种粒子群可以稳定共存,这一发现将为通过设计生产酪蛋白纳米粒子提供一条途径。
更新日期:2020-10-01
中文翻译:

酪蛋白和大米谷蛋白的相互作用:结合机制和分子组装行为
摘要 本研究调查了酸性酪蛋白和大米谷蛋白(RG)在中性条件下的相互作用。大颗粒群含有酪蛋白-RG复合物,由β-酪蛋白、αs1-酪蛋白组成,形成RG。多光谱研究结果表明,酪蛋白的肽链和二级结构在结合过程中没有发生变化。此外,结合行为似乎与 RG 的表面疏水区密切相关。热力学参数计算表明,这种结构形成背后的主要驱动力是氢键和范德华力。另一方面,RG的加入导致产生主要由κ-酪蛋白和αs2-酪蛋白组成的小颗粒群(~20 nm),并且这种小颗粒群通过残留的钙保持聚集。此外,注意到这两种粒子群可以稳定共存,这一发现将为通过设计生产酪蛋白纳米粒子提供一条途径。







































 京公网安备 11010802027423号
京公网安备 11010802027423号