当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Composition-Dependent Catalytic Activities of Noble-Metal-Free NiS/Ni3S4 for Hydrogen Evolution Reaction
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-07-05 00:00:00 , DOI: 10.1021/acs.jpcc.6b05230
Zhixiao Qin 1 , Yubin Chen 1 , Zhenxiong Huang 1 , Jinzhan Su 1 , Zhidan Diao 1 , Liejin Guo 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2016-07-05 00:00:00 , DOI: 10.1021/acs.jpcc.6b05230
Zhixiao Qin 1 , Yubin Chen 1 , Zhenxiong Huang 1 , Jinzhan Su 1 , Zhidan Diao 1 , Liejin Guo 1
Affiliation
|
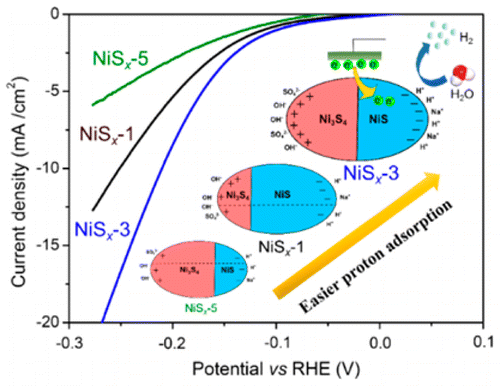
The development of efficient noble-metal-free hydrogen evolution catalysts is quite appealing with the aim of providing cost-competitive hydrogen. Herein, nickel sulfides (NiSx) with tunable NiS/Ni3S4 molar ratios were synthesized via a simple hydrothermal method. Detailed electrochemical studies under neutral conditions indicated that the electrocatalytic property of NiSx catalysts was determined by the composition. Notably, the NiSx sample with the NiS/Ni3S4 molar ratio of 1.0 exhibited the lowest overpotential and charge-transfer resistance. As analyzed from the Tafel plots, the rate-determining step of NiSx catalysts for hydrogen generation was the Volmer step, in which the proton adsorption played a key role. Theoretical calculation revealed that NiS and Ni3S4 exhibited the metallic behaviors with different work functions. Consequently, the NiSx sample with the NiS/Ni3S4 molar ratio of 1.0 owned the most adsorbed protons, which led to the highest electrocatalytic property. Meanwhile, NiSx was demonstrated to be efficient cocatalysts to promote photocatalytic hydrogen generation. NiSx/CdS with the NiS/Ni3S4 molar ratio of 1.0 showed the best photocatalytic activity with the apparent quantum efficiency of 56.5% at 420 nm. This result was in good agreement with the electrocatalytic activities of NiSx samples, indicating the intrinsic property for efficient hydrogen generation.
中文翻译:

无贵金属的NiS / Ni 3 S 4的组分依赖性催化氢分解反应的活性
为了提供具有成本竞争力的氢,开发有效的不含贵金属的氢析出催化剂是非常有吸引力的。在此,通过简单的水热法合成了具有可调的NiS / Ni 3 S 4摩尔比的硫化镍(NiS x)。在中性条件下进行的详细电化学研究表明,NiS x催化剂的电催化性能取决于其组成。值得注意的是,NiS / Ni 3 S 4摩尔比为1.0的NiS x样品表现出最低的过电势和电荷转移阻力。根据塔菲尔曲线分析,NiS x的速率确定步骤氢气产生的催化剂是沃尔默(Volmer)步骤,质子吸附起着关键作用。理论计算表明,NiS和Ni 3 S 4在不同的功函数下表现出金属行为。因此,NiS / Ni 3 S 4摩尔比为1.0的NiS x样品具有最大的吸附质子,从而导致最高的电催化性能。同时,NiS x被证明是促进光催化氢生成的有效助催化剂。NiS x / CdS与NiS / Ni 3 S 4摩尔比为1.0时表现出最佳的光催化活性,在420 nm处的表观量子效率为56.5%。该结果与NiS x样品的电催化活性高度吻合,表明了高效氢生成的内在特性。
更新日期:2016-07-05
中文翻译:

无贵金属的NiS / Ni 3 S 4的组分依赖性催化氢分解反应的活性
为了提供具有成本竞争力的氢,开发有效的不含贵金属的氢析出催化剂是非常有吸引力的。在此,通过简单的水热法合成了具有可调的NiS / Ni 3 S 4摩尔比的硫化镍(NiS x)。在中性条件下进行的详细电化学研究表明,NiS x催化剂的电催化性能取决于其组成。值得注意的是,NiS / Ni 3 S 4摩尔比为1.0的NiS x样品表现出最低的过电势和电荷转移阻力。根据塔菲尔曲线分析,NiS x的速率确定步骤氢气产生的催化剂是沃尔默(Volmer)步骤,质子吸附起着关键作用。理论计算表明,NiS和Ni 3 S 4在不同的功函数下表现出金属行为。因此,NiS / Ni 3 S 4摩尔比为1.0的NiS x样品具有最大的吸附质子,从而导致最高的电催化性能。同时,NiS x被证明是促进光催化氢生成的有效助催化剂。NiS x / CdS与NiS / Ni 3 S 4摩尔比为1.0时表现出最佳的光催化活性,在420 nm处的表观量子效率为56.5%。该结果与NiS x样品的电催化活性高度吻合,表明了高效氢生成的内在特性。







































 京公网安备 11010802027423号
京公网安备 11010802027423号