当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tris(2-pyridyl) Bismuthines: Coordination Chemistry, Reactivity, and Anion-Triggered Pyridyl Coupling.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-04-24 , DOI: 10.1021/acs.inorgchem.0c00579 Álvaro García-Romero 1 , Alex J Plajer 2 , Daniel Miguel 1 , Dominic S Wright 2 , Andrew D Bond 2 , Celedonio M Álvarez 1 , Raúl García-Rodríguez 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-04-24 , DOI: 10.1021/acs.inorgchem.0c00579 Álvaro García-Romero 1 , Alex J Plajer 2 , Daniel Miguel 1 , Dominic S Wright 2 , Andrew D Bond 2 , Celedonio M Álvarez 1 , Raúl García-Rodríguez 1
Affiliation
|
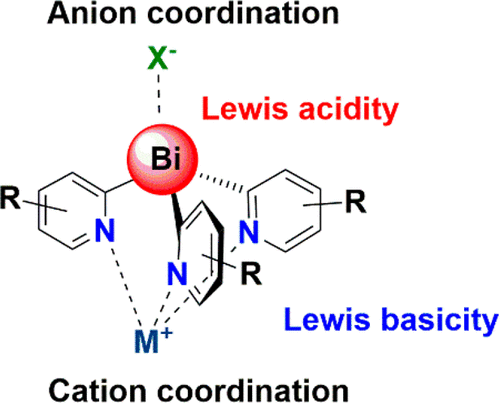
A series of new tris(2-pyridyl) bismuthine ligands of the type [Bi(2-py')3] have been prepared, containing a range of substituents at various positions within their pyridyl rings (py'). They can act as intact ligands or, as a result of the low C-Bi bond energy, exhibit noninnocent reactivity in the presence of metal ions. Structural studies of Li+ and Ag+ complexes show that the coordination to metal ions using their pyridyl-N atoms and to anions using the Lewis acidity of their Bi(III) centers can be modified by the presence of substituents within the 2-pyridyl rings, especially at the 6- or 3-positions, which can block the donor-N or Lewis acid Bi sites. Electron withdrawing groups (like CF3 or Br) can also severely reduce their ability to act as ligands to metal ions by reducing the electron donating ability of the pyridyl-N atoms. Noninnocent character is found in the reactions with Cu+ and Cu2+, resulting in the coupling of pyridyl groups to form bipyridines, with the rate of this reaction being dependent on the anion present in the metal salts. This leads to the formation of Bi(III)/Cu(I) complexes containing hypervalent [X2Bi(2-R-py)]- (X = Cl, Br) anions. Alternatively, the tris(2-pyridyl) bismuthine ligands can act as 2-pyridyl transfer reagents, transferring 2-py groups to Au(I) and Fe(II).
中文翻译:

三(2-吡啶基)铋:配位化学,反应性和阴离子引发的吡啶基偶联。
制备了一系列新的[Bi(2-py')3]型三(2-吡啶基)铋配位体,在其吡啶基环(py')的各个位置上含有一系列取代基。它们可以充当完整的配体,或由于低C-Bi键能而在金属离子存在下显示出无害的反应性。Li +和Ag +配合物的结构研究表明,利用吡啶基-N原子与金属离子的配位以及利用Bi(III)中心的路易斯酸度对阴离子的配位可以通过2-吡啶基环内的取代基的存在来修饰。在6-或3-位上,其可以阻断供体-N或路易斯酸Bi位点。吸电子基团(如CF3或Br)还可以通过降低吡啶基N原子的供电子能力来严重降低其作为金属离子配体的能力。在与Cu +和Cu2 +的反应中发现无毒的特征,导致吡啶基偶联形成联吡啶,该反应的速率取决于金属盐中存在的阴离子。这导致形成含有高价[X2Bi(2-R-py)]-(X = Cl,Br)阴离子的Bi(III)/ Cu(I)络合物。或者,三(2-吡啶基)铋配体可以充当2-吡啶基转移试剂,将2-py基转移到Au(I)和Fe(II)上。
更新日期:2020-04-24
中文翻译:

三(2-吡啶基)铋:配位化学,反应性和阴离子引发的吡啶基偶联。
制备了一系列新的[Bi(2-py')3]型三(2-吡啶基)铋配位体,在其吡啶基环(py')的各个位置上含有一系列取代基。它们可以充当完整的配体,或由于低C-Bi键能而在金属离子存在下显示出无害的反应性。Li +和Ag +配合物的结构研究表明,利用吡啶基-N原子与金属离子的配位以及利用Bi(III)中心的路易斯酸度对阴离子的配位可以通过2-吡啶基环内的取代基的存在来修饰。在6-或3-位上,其可以阻断供体-N或路易斯酸Bi位点。吸电子基团(如CF3或Br)还可以通过降低吡啶基N原子的供电子能力来严重降低其作为金属离子配体的能力。在与Cu +和Cu2 +的反应中发现无毒的特征,导致吡啶基偶联形成联吡啶,该反应的速率取决于金属盐中存在的阴离子。这导致形成含有高价[X2Bi(2-R-py)]-(X = Cl,Br)阴离子的Bi(III)/ Cu(I)络合物。或者,三(2-吡啶基)铋配体可以充当2-吡啶基转移试剂,将2-py基转移到Au(I)和Fe(II)上。































 京公网安备 11010802027423号
京公网安备 11010802027423号