当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Knockdown of milk-fat globule EGF factor-8 suppresses glioma progression in GL261 glioma cells by repressing microglial M2 polarization.
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-04-23 , DOI: 10.1002/jcp.29712 Jing Wu 1 , Huicui Yang 1 , Junjie Cheng 1 , Li Zhang 1 , Youliang Ke 1 , Yi Zhu 1 , Cheng Wang 2 , Xiaohu Zhang 1 , Xuechu Zhen 1 , Long Tai Zheng 1
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-04-23 , DOI: 10.1002/jcp.29712 Jing Wu 1 , Huicui Yang 1 , Junjie Cheng 1 , Li Zhang 1 , Youliang Ke 1 , Yi Zhu 1 , Cheng Wang 2 , Xiaohu Zhang 1 , Xuechu Zhen 1 , Long Tai Zheng 1
Affiliation
|
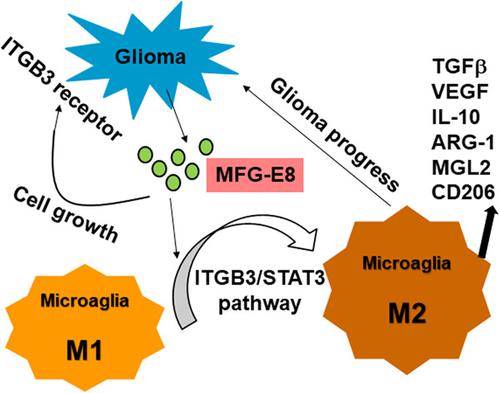
Tumor‐associated microglial cells promote glioma growth, invasion, and chemoresistance by releasing inflammatory factors. Milk fat globule EGF factor 8 protein (MFG‐E8), a secreted glycoprotein, is closely related to tissue homeostasis and anti‐inflammation. In the present study, we investigated the role of MFG‐E8 in microglial polarization and glioma progression in vitro and in vivo. We found that glioma cells secrete comparable amounts of MFG‐E8 in culture media to astrocytes. Recombinant MFG‐E8 triggered microglia to express the M2 polarization markers, such as arginase‐1 (ARG‐1), macrophage galactose‐type C‐type lectin‐2 (MGL‐2), and macrophage mannose receptor (CD206). Forced expression of MFG‐E8 in BV‐2 microglia cells not only promoted IL‐4‐induced M2 polarization but also inhibited lipopolysaccharide (LPS)‐induced M1 microglial polarization. Mechanistic studies demonstrated that recombinant MFG‐E8 markedly induced signal transducer and activator of transcription 3 (STAT3) phosphorylation, and the STAT3 inhibitor stattic significantly blocked MFG‐E8‐induced ARG‐1 expression. Administration of antibody against MFG‐E8 and knockdown of its receptor, integrin β3, significantly attenuated MFG‐E8‐induced ARG‐1 expression. Similarly, knockdown of MFG‐E8 also markedly reduced IL‐4‐induced M2 marker expression and increased LPS‐induced M1 marker expression in microglia cells. Moreover, the knockdown of MFG‐E8 in GL261 glioma cells inhibited cell proliferation and enhanced chemosensitivity to 1,3‐bis(2‐chloroethyl)‐1‐nitrosourea (BCNU), which was likely associated with the downregulation of FAK/AKT activation and STAT3/cyclin D1 signaling. The murine GL261 glioma experimental model demonstrated that knockdown of MFG‐E8 significantly reduced tumor size and extended survival times. Additionally, attenuated CD11b+ cell infiltration and reduced CD206+ expression in CD11b+ cells were also observed in an MFG‐E8 knockdown GL261 murine glioma model. These results suggested that inhibition of MFG‐E8 might hamper the immunosuppressive microenvironment in gliomas and therefore ameliorate tumor progression.
中文翻译:

抑制乳脂小球EGF因子8通过抑制小胶质细胞M2极化来抑制GL261胶质瘤细胞中的胶质瘤进展。
肿瘤相关的小胶质细胞通过释放炎症因子来促进神经胶质瘤的生长,侵袭和化学抗性。乳脂球EGF因子8蛋白(MFG-E8)是一种分泌的糖蛋白,与组织稳态和抗炎性密切相关。在本研究中,我们研究了MFG-E8在体外和体内在小胶质细胞极化和神经胶质瘤进展中的作用。。我们发现神经胶质瘤细胞在培养基中分泌与星形胶质细胞相当量的MFG-E8。重组MFG-E8触发小胶质细胞表达M2极化标记,例如精氨酸酶-1(ARG-1),巨噬细胞半乳糖C型凝集素2(MGL-2)和巨噬细胞甘露糖受体(CD206)。在BV-2小胶质细胞中强迫表达MFG-E8不仅促进IL-4诱导的M2极化,而且抑制脂多糖(LPS)诱导的M1小胶质细胞极化。机理研究表明,重组MFG-E8明显诱导了信号转导子和转录激活因子3(STAT3)的磷酸化,而STAT3抑制剂则明显阻断了MFG-E8诱导的ARG-1的表达。施用针对MFG-E8的抗体及其受体整合素β3的敲低显着减弱了MFG-E8诱导的ARG-1的表达。同样,在小胶质细胞中,MFG-E8的敲除也显着降低了IL-4诱导的M2标志表达,并增加了LPS诱导的M1标志表达。此外,在GL261胶质瘤细胞中敲低MFG-E8抑制了细胞增殖并增强了对1,3-双(2-氯乙基)-1-亚硝基脲(BCNU)的化学敏感性,这可能与FAK / AKT激活的下调和STAT3 /细胞周期蛋白D1信号传导。小鼠GL261神经胶质瘤实验模型表明,敲除MFG-E8可以显着减小肿瘤大小并延长生存时间。此外,衰减的CD11b GL261神经胶质瘤细胞中MFG-E8的抑制可抑制细胞增殖并增强对1,3-双(2-氯乙基)-1-亚硝基脲(BCNU)的化学敏感性,这可能与FAK / AKT激活和STAT3 /的下调有关细胞周期蛋白D1信号传导。小鼠GL261神经胶质瘤实验模型表明,敲除MFG-E8可以显着减小肿瘤大小并延长生存时间。此外,衰减的CD11b GL261神经胶质瘤细胞中MFG-E8的抑制可抑制细胞增殖并增强对1,3-双(2-氯乙基)-1-亚硝基脲(BCNU)的化学敏感性,这可能与FAK / AKT激活和STAT3 /的下调有关细胞周期蛋白D1信号传导。小鼠GL261神经胶质瘤实验模型表明,敲除MFG-E8可以显着减小肿瘤大小并延长生存时间。此外,衰减的CD11b在MFG-E8基因敲除的GL261鼠神经胶质瘤模型中也观察到+细胞浸润和CD11b +细胞CD206 +表达减少。这些结果表明,对MFG-E8的抑制可能会阻碍神经胶质瘤的免疫抑制微环境,从而改善肿瘤的进展。
更新日期:2020-04-23
中文翻译:

抑制乳脂小球EGF因子8通过抑制小胶质细胞M2极化来抑制GL261胶质瘤细胞中的胶质瘤进展。
肿瘤相关的小胶质细胞通过释放炎症因子来促进神经胶质瘤的生长,侵袭和化学抗性。乳脂球EGF因子8蛋白(MFG-E8)是一种分泌的糖蛋白,与组织稳态和抗炎性密切相关。在本研究中,我们研究了MFG-E8在体外和体内在小胶质细胞极化和神经胶质瘤进展中的作用。。我们发现神经胶质瘤细胞在培养基中分泌与星形胶质细胞相当量的MFG-E8。重组MFG-E8触发小胶质细胞表达M2极化标记,例如精氨酸酶-1(ARG-1),巨噬细胞半乳糖C型凝集素2(MGL-2)和巨噬细胞甘露糖受体(CD206)。在BV-2小胶质细胞中强迫表达MFG-E8不仅促进IL-4诱导的M2极化,而且抑制脂多糖(LPS)诱导的M1小胶质细胞极化。机理研究表明,重组MFG-E8明显诱导了信号转导子和转录激活因子3(STAT3)的磷酸化,而STAT3抑制剂则明显阻断了MFG-E8诱导的ARG-1的表达。施用针对MFG-E8的抗体及其受体整合素β3的敲低显着减弱了MFG-E8诱导的ARG-1的表达。同样,在小胶质细胞中,MFG-E8的敲除也显着降低了IL-4诱导的M2标志表达,并增加了LPS诱导的M1标志表达。此外,在GL261胶质瘤细胞中敲低MFG-E8抑制了细胞增殖并增强了对1,3-双(2-氯乙基)-1-亚硝基脲(BCNU)的化学敏感性,这可能与FAK / AKT激活的下调和STAT3 /细胞周期蛋白D1信号传导。小鼠GL261神经胶质瘤实验模型表明,敲除MFG-E8可以显着减小肿瘤大小并延长生存时间。此外,衰减的CD11b GL261神经胶质瘤细胞中MFG-E8的抑制可抑制细胞增殖并增强对1,3-双(2-氯乙基)-1-亚硝基脲(BCNU)的化学敏感性,这可能与FAK / AKT激活和STAT3 /的下调有关细胞周期蛋白D1信号传导。小鼠GL261神经胶质瘤实验模型表明,敲除MFG-E8可以显着减小肿瘤大小并延长生存时间。此外,衰减的CD11b GL261神经胶质瘤细胞中MFG-E8的抑制可抑制细胞增殖并增强对1,3-双(2-氯乙基)-1-亚硝基脲(BCNU)的化学敏感性,这可能与FAK / AKT激活和STAT3 /的下调有关细胞周期蛋白D1信号传导。小鼠GL261神经胶质瘤实验模型表明,敲除MFG-E8可以显着减小肿瘤大小并延长生存时间。此外,衰减的CD11b在MFG-E8基因敲除的GL261鼠神经胶质瘤模型中也观察到+细胞浸润和CD11b +细胞CD206 +表达减少。这些结果表明,对MFG-E8的抑制可能会阻碍神经胶质瘤的免疫抑制微环境,从而改善肿瘤的进展。































 京公网安备 11010802027423号
京公网安备 11010802027423号