当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4-Nitrophenol Reduction: Probing the Putative Mechanism of the Model Reaction
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-04-23 , DOI: 10.1021/acscatal.0c00725
Jyah Strachan 1 , Christopher Barnett 1 , Anthony F. Masters 1 , Thomas Maschmeyer 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-04-23 , DOI: 10.1021/acscatal.0c00725
Jyah Strachan 1 , Christopher Barnett 1 , Anthony F. Masters 1 , Thomas Maschmeyer 1
Affiliation
|
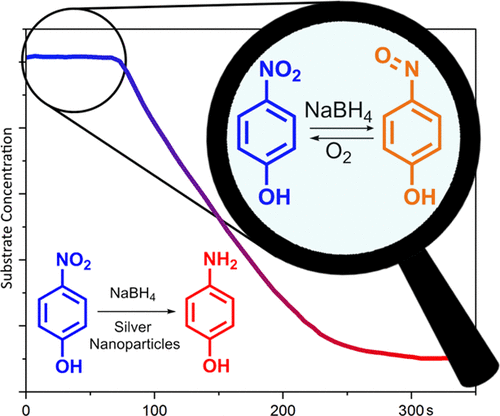
We report a reinterpretation of the reduction of 4-nitrophenol catalyzed by silver nanoparticles. Mass spectrometry and ultraviolet–visible light spectroscopy measurements support the existence of 4-nitrosophenol as a stable reaction intermediate. We propose that dissolved oxygen is consumed, both by oxidizing 4-nitrosophenol (an intermediate) and reoxidizing the reduced catalyst surface, resulting in the commonly observed “induction period” in the reaction kinetics. Upon complete consumption of dissolved oxygen, subsequent reduction to 4-aminophenol can occur. A complete kinetic analysis including modeling is presented, conceptually fitting data from recent reports in the literature, as well as fitting data from our own experiments.
中文翻译:

4-硝基苯酚还原:探索模型反应的推定机理
我们报告了银纳米粒子催化的4-硝基苯酚还原的重新解释。质谱法和紫外-可见光光谱法测量结果支持了4-亚硝基苯酚作为稳定的反应中间体的存在。我们建议通过氧化4-亚硝基苯酚(一种中间体)和再氧化还原的催化剂表面来消耗溶解的氧,从而导致反应动力学中通常观察到的“诱导期”。溶解氧完全消耗后,随后可能还原为4-氨基苯酚。提出了包括模型在内的完整动力学分析,概念上是文献中最近报告的数据拟合,也是我们实验中的拟合数据。
更新日期:2020-04-23
中文翻译:

4-硝基苯酚还原:探索模型反应的推定机理
我们报告了银纳米粒子催化的4-硝基苯酚还原的重新解释。质谱法和紫外-可见光光谱法测量结果支持了4-亚硝基苯酚作为稳定的反应中间体的存在。我们建议通过氧化4-亚硝基苯酚(一种中间体)和再氧化还原的催化剂表面来消耗溶解的氧,从而导致反应动力学中通常观察到的“诱导期”。溶解氧完全消耗后,随后可能还原为4-氨基苯酚。提出了包括模型在内的完整动力学分析,概念上是文献中最近报告的数据拟合,也是我们实验中的拟合数据。



































 京公网安备 11010802027423号
京公网安备 11010802027423号