当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Allylic Alkylation of β-Ketoesters via C–N Bond Cleavage of N-Allyl-N-methylaniline Derivatives Catalyzed by a Nickel–Diphosphine System
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-04-22 , DOI: 10.1021/acscatal.0c01356
Haruki Nagae 1 , Jingzhao Xia 1, 2 , Evgueni Kirillov 3 , Kosuke Higashida 1 , Koya Shoji 1 , Valentin Boiteau 1 , Wanbin Zhang 2 , Jean-François Carpentier 3 , Kazushi Mashima 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-04-22 , DOI: 10.1021/acscatal.0c01356
Haruki Nagae 1 , Jingzhao Xia 1, 2 , Evgueni Kirillov 3 , Kosuke Higashida 1 , Koya Shoji 1 , Valentin Boiteau 1 , Wanbin Zhang 2 , Jean-François Carpentier 3 , Kazushi Mashima 1
Affiliation
|
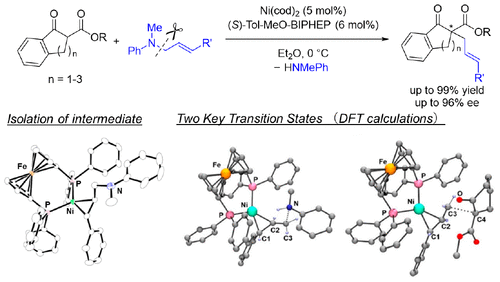
Nickel complexes bearing chiral diphosphine ligands, such as (S)-Tol-MeO-BIPHEP and (S)-H8-BINAP, serve as efficient catalysts for asymmetric allylic alkylation (AAA) of β-ketoesters, using allylic amines as allyl sources. The reactions proceed with high catalytic activity and high enantioselectivity. N-Methyl-N-phenyl allylic amines were indispensable to achieve the high catalytic activity, to achieve the high enantioselectivity, and to expand the substrate scope to 5- and 7-membered β-ketoesters, whose nickel-catalyzed AAA with allylic alcohols results in low enantioselectivity. On the basis of the kinetics using a catalyst system made of Ni(cod)2 and (S)-Tol-MeO-BIPHEP, and DFT calculations for the reaction pathway of the AAA reaction mediated by an isolated olefin-coordinated nickel–DPPF complex 4b, we propose a mechanism where protonation of the nitrogen atom of the coordinating allylic amine by β-ketoester is key to cleaving the C–N bond and delivering a cationic π-allyl nickel(II) intermediate.
中文翻译:

通过的C-N键断裂β酮酯的不对称烯丙基烷基化Ñ -Allyl- Ñ由镍-双膦体系催化甲基苯胺衍生物
带有手性二膦配体的镍配合物,例如(S)-Tol-MeO-BIPHEP和(S)-H 8 -BINAP,使用烯丙基胺作为烯丙基来源,可作为β-酮酸酯不对称烯丙基烷基化(AAA)的有效催化剂。反应以高催化活性和高对映选择性进行。N-甲基-N-苯基烯丙基胺对于实现高催化活性,实现高对映选择性以及将底物范围扩展至5元和7元β-酮酸酯都是必不可少的,其与烯丙基醇的镍催化AAA合成对映选择性低。根据动力学,使用由Ni(cod)2和(S)-Tol-MeO-BIPHEP,以及由分离的烯烃配位的镍-DPPF配合物4b介导的AAA反应的反应路径的DFT计算,我们提出了一种机制,其中配位的烯丙基胺的氮原子被β-酮酸酯是裂解C–N键并提供阳离子π-烯丙基镍(II)中间体的关键。
更新日期:2020-04-22
中文翻译:

通过的C-N键断裂β酮酯的不对称烯丙基烷基化Ñ -Allyl- Ñ由镍-双膦体系催化甲基苯胺衍生物
带有手性二膦配体的镍配合物,例如(S)-Tol-MeO-BIPHEP和(S)-H 8 -BINAP,使用烯丙基胺作为烯丙基来源,可作为β-酮酸酯不对称烯丙基烷基化(AAA)的有效催化剂。反应以高催化活性和高对映选择性进行。N-甲基-N-苯基烯丙基胺对于实现高催化活性,实现高对映选择性以及将底物范围扩展至5元和7元β-酮酸酯都是必不可少的,其与烯丙基醇的镍催化AAA合成对映选择性低。根据动力学,使用由Ni(cod)2和(S)-Tol-MeO-BIPHEP,以及由分离的烯烃配位的镍-DPPF配合物4b介导的AAA反应的反应路径的DFT计算,我们提出了一种机制,其中配位的烯丙基胺的氮原子被β-酮酸酯是裂解C–N键并提供阳离子π-烯丙基镍(II)中间体的关键。

































 京公网安备 11010802027423号
京公网安备 11010802027423号