当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical chemistry in oxidation flow reactors for atmospheric chemistry research.
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2020-04-21 , DOI: 10.1039/c9cs00766k Zhe Peng 1 , Jose L Jimenez
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2020-04-21 , DOI: 10.1039/c9cs00766k Zhe Peng 1 , Jose L Jimenez
Affiliation
|
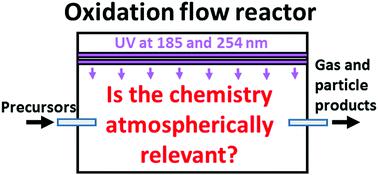
Environmental chambers have been playing a vital role in atmospheric chemistry research for seven decades. In last decade, oxidation flow reactors (OFR) have emerged as a promising alternative to chambers to study complex multigenerational chemistry. OFR can generate higher-than-ambient concentrations of oxidants via H2O, O2 and O3 photolysis by low-pressure-Hg-lamp emissions and reach hours to days of equivalent photochemical aging in just minutes of real time. The use of OFR for volatile-organic-compound (VOC) oxidation and secondary-organic-aerosol formation has grown very rapidly recently. However, the lack of detailed understanding of OFR photochemistry left room for speculation that OFR chemistry may be generally irrelevant to the troposphere, since its initial oxidant generation is similar to stratosphere. Recently, a series of studies have been conducted to address important open questions on OFR chemistry and to guide experimental design and interpretation. In this Review, we present a comprehensive picture connecting the chemistries of hydroxyl (OH) and hydroperoxy radicals, oxidized nitrogen species and organic peroxy radicals (RO2) in OFR. Potential lack of tropospheric relevance associated with these chemistries, as well as the physical conditions resulting in it will also be reviewed. When atmospheric oxidation is dominated by OH, OFR conditions can often be similar to ambient conditions, as OH dominates against undesired non-OH effects. One key reason for tropospherically-irrelevant/undesired VOC fate is that under some conditions, OH is drastically reduced while non-tropospheric/undesired VOC reactants are not. The most frequent problems are running experiments with too high precursor concentrations, too high UV and/or too low humidity. On other hand, another cause of deviation from ambient chemistry in OFR is that some tropospherically-relevant non-OH chemistry (e.g. VOC photolysis in UVA and UVB) is not sufficiently represented under some conditions. In addition, the fate of RO2 produced from VOC oxidation can be kept relevant to the troposphere. However, in some cases RO2 lifetime can be too short for atmospherically-relevant RO2 chemistry, including its isomerization. OFR applications using only photolysis of injected O3 to generate OH are less preferable than those using both 185 and 254 nm photons (without O3 injection) for several reasons. When a relatively low equivalent photochemical age (<∼1 d) and high NO are needed, OH and NO generation by organic-nitrite photolysis in the UVA range is preferable. We also discuss how to achieve the atmospheric relevance for different purposes in OFR experimental planning.
中文翻译:

用于大气化学研究的氧化流反应器中的自由基化学。
七十年来,环境室在大气化学研究中一直发挥着至关重要的作用。在过去的十年中,氧化流反应器(OFR)成为研究复杂多代化学反应室的有前途的替代方法。OFR可以通过低压Hg灯的辐射通过H2O,O2和O3的光解产生高于环境浓度的氧化剂,并在短短的几分钟内达到数小时至数天的等效光化学老化时间。近来,OFR在挥发性有机化合物(VOC)氧化和次要有机气溶胶形成中的应用增长非常迅速。然而,由于缺乏对OFR光化学的详细了解,因此人们推测OFR化学通常与对流层无关,这是因为它的初始氧化剂生成与平流层相似,因此尚无定论。最近,已经进行了一系列研究,以解决关于OFR化学的重要开放问题,并指导实验设计和解释。在这篇综述中,我们呈现了一个综合的图景,连接了OFR中的羟基(OH)和氢过氧自由基,氧化的氮物种和有机过氧自由基(RO2)的化学性质。与这些化学物质有关的潜在对流层相关性缺乏,以及由此产生的物理条件也将得到审查。当大气氧化主要由OH决定时,OFR条件通常类似于环境条件,因为OH相对于不希望的非OH效应起主要作用。对流层无关/不想要的VOC归因的一个关键原因是,在某些条件下,OH会急剧减少,而非对流层/不想要的VOC反应物却不会。最常见的问题是在过高的前驱物浓度,过高的UV和/或过低的湿度下进行实验。另一方面,OFR中偏离环境化学的另一个原因是,在某些条件下,与对流层相关的非OH化学(例如,UVA和UVB中的VOC光解)没有得到充分体现。另外,VOC氧化产生的RO2的命运可以保持与对流层相关。但是,在某些情况下,对于大气相关的RO2化学物质(包括其异构化),RO2的寿命可能太短。由于一些原因,仅使用注入的O3进行光解以生成OH的OFR应用比使用185和254 nm光子(不注入O3)的应用更不受欢迎。当需要较低的等效光化学年龄(<〜1 d)和较高的NO时,在UVA范围内通过有机亚硝酸盐光解产生OH和NO是优选的。我们还将讨论在OFR实验计划中如何实现不同目的的大气相关性。
更新日期:2020-04-21
中文翻译:

用于大气化学研究的氧化流反应器中的自由基化学。
七十年来,环境室在大气化学研究中一直发挥着至关重要的作用。在过去的十年中,氧化流反应器(OFR)成为研究复杂多代化学反应室的有前途的替代方法。OFR可以通过低压Hg灯的辐射通过H2O,O2和O3的光解产生高于环境浓度的氧化剂,并在短短的几分钟内达到数小时至数天的等效光化学老化时间。近来,OFR在挥发性有机化合物(VOC)氧化和次要有机气溶胶形成中的应用增长非常迅速。然而,由于缺乏对OFR光化学的详细了解,因此人们推测OFR化学通常与对流层无关,这是因为它的初始氧化剂生成与平流层相似,因此尚无定论。最近,已经进行了一系列研究,以解决关于OFR化学的重要开放问题,并指导实验设计和解释。在这篇综述中,我们呈现了一个综合的图景,连接了OFR中的羟基(OH)和氢过氧自由基,氧化的氮物种和有机过氧自由基(RO2)的化学性质。与这些化学物质有关的潜在对流层相关性缺乏,以及由此产生的物理条件也将得到审查。当大气氧化主要由OH决定时,OFR条件通常类似于环境条件,因为OH相对于不希望的非OH效应起主要作用。对流层无关/不想要的VOC归因的一个关键原因是,在某些条件下,OH会急剧减少,而非对流层/不想要的VOC反应物却不会。最常见的问题是在过高的前驱物浓度,过高的UV和/或过低的湿度下进行实验。另一方面,OFR中偏离环境化学的另一个原因是,在某些条件下,与对流层相关的非OH化学(例如,UVA和UVB中的VOC光解)没有得到充分体现。另外,VOC氧化产生的RO2的命运可以保持与对流层相关。但是,在某些情况下,对于大气相关的RO2化学物质(包括其异构化),RO2的寿命可能太短。由于一些原因,仅使用注入的O3进行光解以生成OH的OFR应用比使用185和254 nm光子(不注入O3)的应用更不受欢迎。当需要较低的等效光化学年龄(<〜1 d)和较高的NO时,在UVA范围内通过有机亚硝酸盐光解产生OH和NO是优选的。我们还将讨论在OFR实验计划中如何实现不同目的的大气相关性。































 京公网安备 11010802027423号
京公网安备 11010802027423号