当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Step Dynamic Imine Chemistry for Preparation of Chitosan-Stabilized Emulsions Using a Natural Aldehyde: Acid Trigger Mechanism and Regulation and Gastric Delivery.
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-04-30 , DOI: 10.1021/acs.jafc.9b08301 Huanle Chen 1, 2 , Runan Zhao 1 , Junjie Hu 1 , Zixiang Wei 2 , David Julian McClements 3 , Shilin Liu 1, 4 , Bin Li 1, 4 , Yan Li 1, 4
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-04-30 , DOI: 10.1021/acs.jafc.9b08301 Huanle Chen 1, 2 , Runan Zhao 1 , Junjie Hu 1 , Zixiang Wei 2 , David Julian McClements 3 , Shilin Liu 1, 4 , Bin Li 1, 4 , Yan Li 1, 4
Affiliation
|
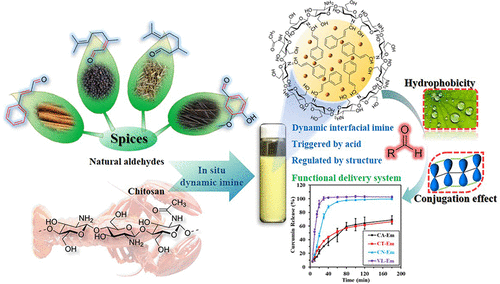
Chitosan is a polysaccharide widely used as a structuring agent in foods and other materials because of its positive charge (amino groups). At present, however, it is difficult to form and stabilize emulsions using chitosan due to its high hydrophilicity. In this study, oil-in-water emulsions were prepared using a one-pot green-chemistry method. The chitosan and aldehyde molecules were in situ interfacially conjugated during homogenization, which promoted the adsorption of chitosan onto the oil droplet surfaces where they created a protective coating. The universality of this method was verified by using chitosan with different molecular weights and four kinds of natural aldehydes [cinnamaldehyde (CA), citral (CT), citronella (CN), and vanillin (VL)]. Chitosan with higher molecular weight facilitated the formation of emulsions. By harnessing the dynamic covalent nature of imine bonds, chitosan emulsions with an imine link display dynamic behavior with acid-catalyzed hydrolysis. The aldehyde structure could control the pH point of trigger for breakdown of emulsions, which was 1.0, 3.0, 4.0, and 4.0 for CA emulsion, CT emulsion, CN emulsion, and VL emulsion, respectively. At pH 6.5, aldehyde helped to decrease the interfacial tension of chitosan to about 10 mN/m, while this value would increase if the pH decreased by adding acid during the measurement. Chemical kinetics studies indicated that the hydrophobicity and conjugation effect of the aldehyde together determined the trigger points and properties of the emulsion. Finally, we used the optimized emulsions to encapsulate and control the release of curcumin. The gastric release behavior of the curcumin depended on aldehyde structure: VL > CN > CT ≈ CA. Hence, a tailor-made trigger release emulsion system can be achieved by rational selection and design of aldehyde structure to control hydrophobicity and conjugation effect of aldehydes.
中文翻译:

使用天然醛制备壳聚糖稳定的乳液的一步式动态亚胺化学:酸触发机制和调节及胃液输送。
壳聚糖是一种多糖,由于其带正电荷(氨基),因此被广泛用作食品和其他材料中的结构剂。然而,目前,由于壳聚糖的高亲水性,难以使用壳聚糖形成和稳定乳液。在这项研究中,使用一锅法绿色化学方法制备水包油乳液。均质化过程中将壳聚糖和醛分子原位界面共轭,这促进了壳聚糖在油滴表面的吸附,从而形成了保护涂层。使用具有不同分子量的壳聚糖和四种天然醛[肉桂醛(CA),柠檬醛(CT),香茅油(CN)和香兰素(VL)]验证了该方法的通用性。具有较高分子量的壳聚糖促进了乳液的形成。通过利用亚胺键的动态共价性质,具有亚胺键的壳聚糖乳液在酸催化水解中表现出动态行为。醛结构可以控制乳剂分解的触发点的pH点,CA乳剂,CT乳剂,CN乳剂和VL乳剂分别为1.0、3.0、4.0和4.0。在pH 6.5时,醛有助于将壳聚糖的界面张力降低至约10 mN / m,而如果在测量过程中通过添加酸降低pH值,则该值会增加。化学动力学研究表明,醛的疏水性和共轭作用共同决定了乳液的触发点和性能。最后,我们使用优化的乳液来封装和控制姜黄素的释放。姜黄素的胃释放行为取决于醛结构:VL> CN> CT≈CA。因此,可以通过合理选择和设计醛结构来控制醛的疏水性和共轭作用,从而获得量身定制的触发释放乳液体系。
更新日期:2020-04-22
中文翻译:

使用天然醛制备壳聚糖稳定的乳液的一步式动态亚胺化学:酸触发机制和调节及胃液输送。
壳聚糖是一种多糖,由于其带正电荷(氨基),因此被广泛用作食品和其他材料中的结构剂。然而,目前,由于壳聚糖的高亲水性,难以使用壳聚糖形成和稳定乳液。在这项研究中,使用一锅法绿色化学方法制备水包油乳液。均质化过程中将壳聚糖和醛分子原位界面共轭,这促进了壳聚糖在油滴表面的吸附,从而形成了保护涂层。使用具有不同分子量的壳聚糖和四种天然醛[肉桂醛(CA),柠檬醛(CT),香茅油(CN)和香兰素(VL)]验证了该方法的通用性。具有较高分子量的壳聚糖促进了乳液的形成。通过利用亚胺键的动态共价性质,具有亚胺键的壳聚糖乳液在酸催化水解中表现出动态行为。醛结构可以控制乳剂分解的触发点的pH点,CA乳剂,CT乳剂,CN乳剂和VL乳剂分别为1.0、3.0、4.0和4.0。在pH 6.5时,醛有助于将壳聚糖的界面张力降低至约10 mN / m,而如果在测量过程中通过添加酸降低pH值,则该值会增加。化学动力学研究表明,醛的疏水性和共轭作用共同决定了乳液的触发点和性能。最后,我们使用优化的乳液来封装和控制姜黄素的释放。姜黄素的胃释放行为取决于醛结构:VL> CN> CT≈CA。因此,可以通过合理选择和设计醛结构来控制醛的疏水性和共轭作用,从而获得量身定制的触发释放乳液体系。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号