当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Roles of Bromine Radicals and Hydroxyl Radicals in the Degradation of Micropollutants by the UV/Bromine Process.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-04-30 , DOI: 10.1021/acs.est.0c00723 Kaiheng Guo 1 , Shanshan Zheng 1, 2 , Xuewen Zhang 1 , Liu Zhao 1 , Shaomin Ji 3 , Chunyan Chen 1 , Zihao Wu 1 , Ding Wang 4 , Jingyun Fang 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-04-30 , DOI: 10.1021/acs.est.0c00723 Kaiheng Guo 1 , Shanshan Zheng 1, 2 , Xuewen Zhang 1 , Liu Zhao 1 , Shaomin Ji 3 , Chunyan Chen 1 , Zihao Wu 1 , Ding Wang 4 , Jingyun Fang 1
Affiliation
|
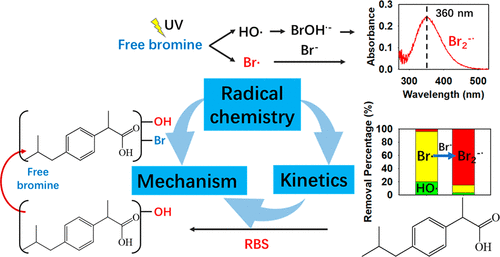
The inevitable occurrence of Br- in natural water affects the degradation kinetics of micropollutants in the UV/chlorine process, the radical chemistry of which, however, is largely unclear. As Br- in the UV/chlorine process first forms free bromine (HOBr/OBr-), this study investigated the radical chemistry of the UV/bromine process for the degradation of selected micropollutants resistant to bromine, i.e., ibuprofen and benzoate, to focus on the roles of radicals. The actual quantum yields of HOBr and OBr- by UV photolysis at 254 nm are 0.43 (±0.025) and 0.26 (±0.025) mol Einstein-1, respectively. Br• and HO• are generated first, and then, Br2•- is formed, with the signal detectable at 360 nm by laser flash photolysis. Compared with Cl• in the UV/chlorine system, Br• exists at higher concentrations (∼10-12 M) in the UV/bromine system while HO• exists at similar concentrations. In the UV/bromine process, reactive bromine species (RBS) dominates the degradation of ibuprofen, while HO• dominates the degradation of benzoate. Br• and Br2•- are reactive toward ibuprofen which second-order rate constants (k) were determined to be 2.2 × 109 and 5.3 × 107 M-1 s-1, respectively, by laser flash photolysis. Br• was the major RBS for ibuprofen degradation by the UV/bromine treatment, whereas Br2•- increasingly contributed to ibuprofen degradation with increasing free bromine or Br- concentrations. Br• could be scavenged by HCO3- and natural organic matter (NOM), and the k with NOM was determined to be 2.6 × 104 (mg/L)-1 s-1. Both Br• and Br2•- prefer to react with ibuprofen via electron transfer with activation energy barriers (Δ‡G0SET) of 1.35 and 7.78 kcal mol-1, respectively. RBS promoted the formation of hydroxylated products. Then free bromine, rather than RBS, was responsible for the formation of brominated products, increasing the total organic bromine (TOBr) and tribromomethane yields in the UV/bromine system. This study demonstrates for the first time the roles of RBS and HO• in micropollutant degradation in the UV/bromine process.
中文翻译:

溴自由基和羟基自由基在紫外/溴过程降解微污染物中的作用。
天然水中不可避免地存在Br-会影响微污染物在UV /氯过程中的降解动力学,但是其自由基化学性质尚不清楚。由于Br-在紫外线/氯过程中首先形成游离溴(HOBr / OBr-),因此本研究调查了紫外线/溴过程中的自由基化学性质,以降解对溴具有抗性的选定微污染物,即布洛芬和苯甲酸盐,关于激进分子的角色。在254 nm处通过UV光解得到的HOBr和OBr-的实际量子产率分别为0.43(±0.025)和0.26(±0.025)mol爱因斯坦-1。首先生成Br•和HO•,然后形成Br2•-,通过激光闪光光解可在360 nm处检测到信号。与紫外线/氯气系统中的Cl•相比,在紫外线/溴系统中,Br•的浓度较高(约10-12 M),而HO•的浓度相似。在紫外线/溴工艺中,反应性溴物质(RBS)在布洛芬的降解中占主导,而HO•在苯甲酸酯的降解中占主导。Br•和Br2•-对布洛芬有反应性,通过激光闪光光解法确定的二级速率常数(k)分别为2.2×109和5.3×107 M-1 s-1。Br•是通过紫外线/溴处理进行布洛芬降解的主要RBS,而Br2-•随着游离溴或Br-浓度的增加而逐渐促进布洛芬降解。Br•可以被HCO3-和天然有机物(NOM)清除,而具有NOM的k被确定为2.6×104(mg / L)-1 s-1。Br•和Br2•-都更喜欢通过电子转移与布洛芬反应,其活化能垒(Δ‡G0SET)分别为1.35和7.78 kcal mol-1。RBS促进了羟基化产物的形成。然后游离的溴而不是RBS负责形成溴化产物,从而增加了UV /溴系统中的有机溴(TOBr)和三溴甲烷的总产量。这项研究首次证明了RBS和HO•在UV /溴过程中的微污染物降解中的作用。
更新日期:2020-04-22
中文翻译:

溴自由基和羟基自由基在紫外/溴过程降解微污染物中的作用。
天然水中不可避免地存在Br-会影响微污染物在UV /氯过程中的降解动力学,但是其自由基化学性质尚不清楚。由于Br-在紫外线/氯过程中首先形成游离溴(HOBr / OBr-),因此本研究调查了紫外线/溴过程中的自由基化学性质,以降解对溴具有抗性的选定微污染物,即布洛芬和苯甲酸盐,关于激进分子的角色。在254 nm处通过UV光解得到的HOBr和OBr-的实际量子产率分别为0.43(±0.025)和0.26(±0.025)mol爱因斯坦-1。首先生成Br•和HO•,然后形成Br2•-,通过激光闪光光解可在360 nm处检测到信号。与紫外线/氯气系统中的Cl•相比,在紫外线/溴系统中,Br•的浓度较高(约10-12 M),而HO•的浓度相似。在紫外线/溴工艺中,反应性溴物质(RBS)在布洛芬的降解中占主导,而HO•在苯甲酸酯的降解中占主导。Br•和Br2•-对布洛芬有反应性,通过激光闪光光解法确定的二级速率常数(k)分别为2.2×109和5.3×107 M-1 s-1。Br•是通过紫外线/溴处理进行布洛芬降解的主要RBS,而Br2-•随着游离溴或Br-浓度的增加而逐渐促进布洛芬降解。Br•可以被HCO3-和天然有机物(NOM)清除,而具有NOM的k被确定为2.6×104(mg / L)-1 s-1。Br•和Br2•-都更喜欢通过电子转移与布洛芬反应,其活化能垒(Δ‡G0SET)分别为1.35和7.78 kcal mol-1。RBS促进了羟基化产物的形成。然后游离的溴而不是RBS负责形成溴化产物,从而增加了UV /溴系统中的有机溴(TOBr)和三溴甲烷的总产量。这项研究首次证明了RBS和HO•在UV /溴过程中的微污染物降解中的作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号