当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt (II)-Catalyzed Stereoselective Olefin Isomerization: Facile Access to Acyclic Trisubstituted Alkenes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-04-21 , DOI: 10.1021/jacs.0c02101 Sheng Zhang 1 , Deepika Bedi 1 , Lu Cheng 1 , Daniel K Unruh 1 , Guigen Li 1 , Michael Findlater 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-04-21 , DOI: 10.1021/jacs.0c02101 Sheng Zhang 1 , Deepika Bedi 1 , Lu Cheng 1 , Daniel K Unruh 1 , Guigen Li 1 , Michael Findlater 1
Affiliation
|
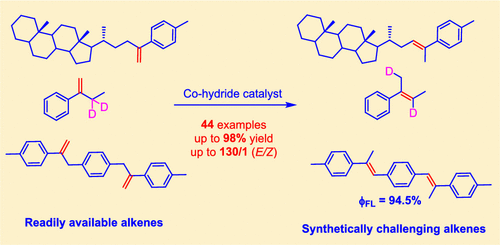
Stereoselective synthesis of trisubstituted alkenes is a long-standing challenge in organic chemistry, due to the small energy differences between E and Z isomers of trisubstituted alkenes (compared with 1,2-disubstituted alkenes). Transition metal-catalyzed isomerization of 1,1-disubstituted alkene can serve as an alternative approach to trisubsti-tuted alkenes, but it remains underdeveloped owing to issues relating to reaction efficiency and stereoselectivity. Here we show a novel cobalt catalyst can overcome these challenges to provide an efficient and stereoselective access to a broad range of trisubstituted alkenes. This protocol is compatible with both mono- and dienes and exhibits a good functional group tolerance and scalability. Moreover, it has proven to be a useful tool to construct organic lumi-nophores and a deuterated trisubstituted-alkene. A preliminary study of the mechanism suggests a cobalt-hydride pathway is involved in the reaction. The high stereoselectivity of the reaction is attributed to both a π-π stacking effect and the steric hindrance between substrate and catalyst.
中文翻译:

钴 (II) 催化立体选择性烯烃异构化:轻松获得无环三取代烯烃
三取代烯烃的立体选择性合成是有机化学中长期存在的挑战,因为三取代烯烃的 E 和 Z 异构体之间的能量差异很小(与 1,2-二取代烯烃相比)。1,1-二取代烯烃的过渡金属催化异构化可以作为三取代烯烃的替代方法,但由于与反应效率和立体选择性有关的问题,它仍然不发达。在这里,我们展示了一种新型钴催化剂可以克服这些挑战,为获得广泛的三取代烯烃提供有效和立体选择性的途径。该协议与单烯和二烯兼容,并表现出良好的官能团耐受性和可扩展性。而且,它已被证明是构建有机发光体和氘代三取代烯烃的有用工具。对该机制的初步研究表明,该反应涉及钴氢化物途径。该反应的高立体选择性归因于 π-π 堆积效应和底物和催化剂之间的空间位阻。
更新日期:2020-04-21
中文翻译:

钴 (II) 催化立体选择性烯烃异构化:轻松获得无环三取代烯烃
三取代烯烃的立体选择性合成是有机化学中长期存在的挑战,因为三取代烯烃的 E 和 Z 异构体之间的能量差异很小(与 1,2-二取代烯烃相比)。1,1-二取代烯烃的过渡金属催化异构化可以作为三取代烯烃的替代方法,但由于与反应效率和立体选择性有关的问题,它仍然不发达。在这里,我们展示了一种新型钴催化剂可以克服这些挑战,为获得广泛的三取代烯烃提供有效和立体选择性的途径。该协议与单烯和二烯兼容,并表现出良好的官能团耐受性和可扩展性。而且,它已被证明是构建有机发光体和氘代三取代烯烃的有用工具。对该机制的初步研究表明,该反应涉及钴氢化物途径。该反应的高立体选择性归因于 π-π 堆积效应和底物和催化剂之间的空间位阻。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号