Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Imbalance of Excitatory/Inhibitory Neuron Differentiation in Neurodevelopmental Disorders with an NR2F1 Point Mutation.
Cell Reports ( IF 7.5 ) Pub Date : 2020-04-21 , DOI: 10.1016/j.celrep.2020.03.085
Ke Zhang 1 , Fang Yu 1 , Jian Zhu 2 , Sue Han 3 , Jiehui Chen 1 , Xuanyuan Wu 4 , Yingying Chen 1 , Tingyu Shen 3 , Jiaoyang Liao 1 , Wenke Guo 1 , Xianfa Yang 5 , Ran Wang 1 , Yun Qian 1 , Jiaxin Yang 2 , Leping Cheng 6 , Yun Zhao 1 , Chi-Chung Hui 7 , Jinsong Li 1 , Guangdun Peng 8 , Shuijin He 4 , Naihe Jing 9 , Ke Tang 2
Cell Reports ( IF 7.5 ) Pub Date : 2020-04-21 , DOI: 10.1016/j.celrep.2020.03.085
Ke Zhang 1 , Fang Yu 1 , Jian Zhu 2 , Sue Han 3 , Jiehui Chen 1 , Xuanyuan Wu 4 , Yingying Chen 1 , Tingyu Shen 3 , Jiaoyang Liao 1 , Wenke Guo 1 , Xianfa Yang 5 , Ran Wang 1 , Yun Qian 1 , Jiaxin Yang 2 , Leping Cheng 6 , Yun Zhao 1 , Chi-Chung Hui 7 , Jinsong Li 1 , Guangdun Peng 8 , Shuijin He 4 , Naihe Jing 9 , Ke Tang 2
Affiliation
|
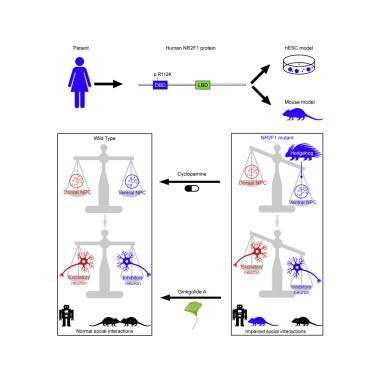
Recent studies have revealed an essential role for embryonic cortical development in the pathophysiology of neurodevelopmental disorders, including autism spectrum disorder (ASD). However, the genetic basis and underlying mechanisms remain unclear. Here, we generate mutant human embryonic stem cell lines (Mut hESCs) carrying an NR2F1-R112K mutation that has been identified in a patient with ASD features and investigate their neurodevelopmental alterations. Mut hESCs overproduce ventral telencephalic neuron progenitors (ventral NPCs) and underproduce dorsal NPCs, causing the imbalance of excitatory/inhibitory neurons. These alterations can be mainly attributed to the aberrantly activated Hedgehog signaling pathway. Moreover, the corresponding Nr2f1 point-mutant mice display a similar excitatory/inhibitory neuron imbalance and abnormal behaviors. Antagonizing the increased inhibitory synaptic transmission partially alleviates their behavioral deficits. Together, our results suggest that the NR2F1-dependent imbalance of excitatory/inhibitory neuron differentiation caused by the activated Hedgehog pathway is one precursor of neurodevelopmental disorders and may enlighten the therapeutic approaches.
中文翻译:

NR2F1点突变在神经发育障碍中的兴奋性/抑制性神经元分化失衡。
最近的研究揭示了胚胎皮层发育在神经发育障碍(包括自闭症谱系障碍(ASD))的病理生理中的重要作用。但是,遗传基础和潜在机制仍不清楚。在这里,我们生成带有NR2F1-R112K突变的突变人类胚胎干细胞系(Mut hESCs),该突变已在具有ASD特征的患者中得到鉴定,并研究了它们的神经发育改变。Mut hESCs产生腹侧脑神经元祖细胞(腹侧NPC),而产生背侧NPC细胞不足,从而引起兴奋性/抑制性神经元失衡。这些改变可主要归因于异常激活的刺猬信号通路。此外,相应的Nr2f1点突变小鼠表现出类似的兴奋性/抑制性神经元失衡和异常行为。拮抗增加的抑制性突触传递可部分缓解其行为缺陷。在一起,我们的结果表明,由激活的刺猬通路引起的兴奋性/抑制性神经元分化的NR2F1依赖性失衡是神经发育障碍的前兆,并可能启发治疗方法。
更新日期:2020-04-21
中文翻译:

NR2F1点突变在神经发育障碍中的兴奋性/抑制性神经元分化失衡。
最近的研究揭示了胚胎皮层发育在神经发育障碍(包括自闭症谱系障碍(ASD))的病理生理中的重要作用。但是,遗传基础和潜在机制仍不清楚。在这里,我们生成带有NR2F1-R112K突变的突变人类胚胎干细胞系(Mut hESCs),该突变已在具有ASD特征的患者中得到鉴定,并研究了它们的神经发育改变。Mut hESCs产生腹侧脑神经元祖细胞(腹侧NPC),而产生背侧NPC细胞不足,从而引起兴奋性/抑制性神经元失衡。这些改变可主要归因于异常激活的刺猬信号通路。此外,相应的Nr2f1点突变小鼠表现出类似的兴奋性/抑制性神经元失衡和异常行为。拮抗增加的抑制性突触传递可部分缓解其行为缺陷。在一起,我们的结果表明,由激活的刺猬通路引起的兴奋性/抑制性神经元分化的NR2F1依赖性失衡是神经发育障碍的前兆,并可能启发治疗方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号