当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
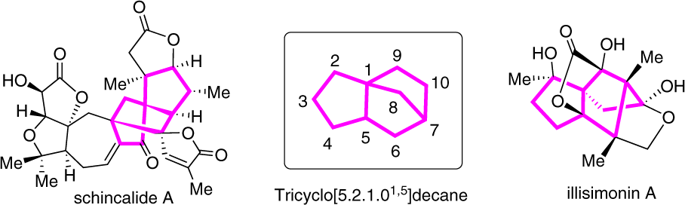
Synthesis of bridged tricyclo[5.2.1.01,5]decanes via nickel-catalyzed asymmetric domino cyclization of enynones.
Nature Communications ( IF 14.7 ) Pub Date : 2020-04-20 , DOI: 10.1038/s41467-020-15837-1 Jiachang Chen 1 , Yiming Wang 1 , Zhengtian Ding 1 , Wangqing Kong 1
Nature Communications ( IF 14.7 ) Pub Date : 2020-04-20 , DOI: 10.1038/s41467-020-15837-1 Jiachang Chen 1 , Yiming Wang 1 , Zhengtian Ding 1 , Wangqing Kong 1
Affiliation
|
The restricted availability, expense and toxicity of precious metal catalysts such as rhodium and palladium challenge the sustainability of synthetic chemistry. As such, nickel catalysts have garnered increasing attention as replacements for enyne cyclization reactions. On the other hand, bridged tricyclo[5.2.1.01,5]decanes are found as core structures in many biologically active natural products; however, the synthesis of such frameworks with high functionalities from readily available precursors remains a significant challenge. Herein, we report a nickel-catalyzed asymmetric domino cyclization reaction of enynones, providing rapid and modular synthesis of bridged tricyclo[5.2.1.01,5]decane skeletons with three quaternary stereocenters in good yields and remarkable high levels of regio- and enantioselectivities (92-99% ee).
中文翻译:

通过镍催化烯酮的不对称多米诺环化合成桥联三环[5.2.1.01,5]癸烷。
铑和钯等贵金属催化剂的可用性、费用和毒性有限,对合成化学的可持续性提出了挑战。因此,镍催化剂作为烯炔环化反应的替代品受到越来越多的关注。另一方面,桥联三环[5.2.1.01,5]癸烷作为许多生物活性天然产物的核心结构被发现;然而,从现成的前体合成具有高功能的框架仍然是一个重大挑战。在此,我们报道了镍催化的烯酮不对称多米诺环化反应,提供了具有三个四元立构中心的桥联三环[5.2.1.01,5]癸烷骨架的快速模块化合成,收率良好,并且具有显着高水平的区域和对映选择性(92 -99% ee)。
更新日期:2020-04-24
中文翻译:

通过镍催化烯酮的不对称多米诺环化合成桥联三环[5.2.1.01,5]癸烷。
铑和钯等贵金属催化剂的可用性、费用和毒性有限,对合成化学的可持续性提出了挑战。因此,镍催化剂作为烯炔环化反应的替代品受到越来越多的关注。另一方面,桥联三环[5.2.1.01,5]癸烷作为许多生物活性天然产物的核心结构被发现;然而,从现成的前体合成具有高功能的框架仍然是一个重大挑战。在此,我们报道了镍催化的烯酮不对称多米诺环化反应,提供了具有三个四元立构中心的桥联三环[5.2.1.01,5]癸烷骨架的快速模块化合成,收率良好,并且具有显着高水平的区域和对映选择性(92 -99% ee)。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号