Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pharmacological and genetic targeting of 5-lipoxygenase interrupts c-Myc oncogenic signaling and kills enzalutamide-resistant prostate cancer cells via apoptosis.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-04-20 , DOI: 10.1038/s41598-020-62845-8 Jitender Monga 1 , Dhatchayini Subramani 1 , Ajay Bharathan 1 , Jagadananda Ghosh 1, 2
Scientific Reports ( IF 3.8 ) Pub Date : 2020-04-20 , DOI: 10.1038/s41598-020-62845-8 Jitender Monga 1 , Dhatchayini Subramani 1 , Ajay Bharathan 1 , Jagadananda Ghosh 1, 2
Affiliation
|
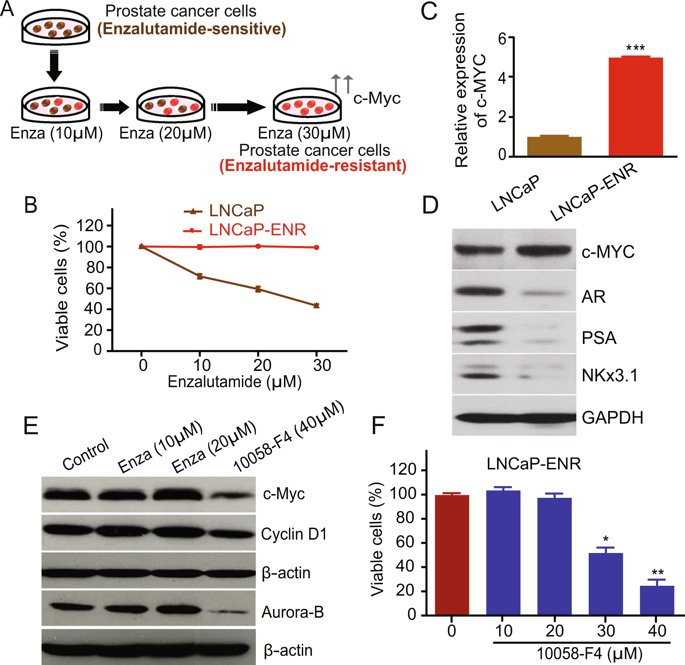
Much of the morbidity and mortality due to prostate cancer happen because of castration-resistant prostate cancer (CRPC) which invariably develops after anti-androgenic therapy. FDA-approved enzalutamide is commonly prescribed for CRPC which works by blocking androgen receptor function. However, even after initial good response, enzalutamide-resistant prostate cancer (ERPC) develops which eventually leads to widespread metastasis. Management of ERPC is extremely difficult because available therapeutic regimen cannot effectively kill and eliminate ERPC cells. Though the mechanism behind enzalutamide-resistance is not properly understood, over-activation of c-Myc has been found to be a common event which plays an important role in the maintenance and progression of ERPC phenotype. However, direct-targeting of c-Myc poses special problem because of its non-enzymatic nature and certain amount of c-Myc activity is needed by non-cancer cells as well. Thus, c-Myc has emerged as an elusive target which needs to be managed by novel agents and strategies in a cancer-specific way. We investigated the effects of pharmacological and genetic inhibition of 5-lipoxygenase (5-Lox) on cell proliferation, apoptosis and invasive potential of enzalutamide-resistant prostate cancer cells. Transcriptional activity of c-Myc was analyzed by DNA-binding, luciferase-assays, and expression of c-Myc-target genes. We found that 5-Lox regulates c-Myc signaling in enzalutamide-resistant prostate cancer cells and inhibition of 5-Lox by Quiflapon/MK591 or shRNA interrupts oncogenic c-Myc signaling and kills ERPC cells by triggering caspase-mediated apoptosis. Interestingly, MK591 does not affect normal, non-cancer cells in the same experimental conditions. Our findings indicate that inhibition of 5-Lox may emerge as a promising new approach to effectively kill ERPC cells sparing normal cells and suggest that development of a long-term curative therapy of prostate cancer may be possible by killing and eliminating ERPC cells with suitable 5-Lox-inhibitors.
中文翻译:

5-脂氧合酶的药理学和遗传靶向可中断 c-Myc 致癌信号传导,并通过细胞凋亡杀死恩杂鲁胺耐药性前列腺癌细胞。
前列腺癌引起的大部分发病率和死亡率都是由去势抵抗性前列腺癌(CRPC)引起的,这种癌总是在抗雄激素治疗后发生。 FDA 批准的恩杂鲁胺通常用于治疗 CRPC,其通过阻断雄激素受体功能发挥作用。然而,即使在最初的良好反应后,恩杂鲁胺耐药性前列腺癌(ERPC)也会发展,最终导致广泛转移。 ERPC的管理极其困难,因为现有的治疗方案无法有效杀死和消除ERPC细胞。尽管恩杂鲁胺耐药性背后的机制尚未得到正确理解,但已发现 c-Myc 过度激活是一种常见事件,在 ERPC 表型的维持和进展中发挥着重要作用。然而,c-Myc 的直接靶向带来了特殊的问题,因为它的非酶性质,并且非癌细胞也需要一定量的 c-Myc 活性。因此,c-Myc 已成为一个难以捉摸的靶点,需要通过新的药物和策略以癌症特异性的方式进行管理。我们研究了 5-脂氧合酶 (5-Lox) 的药理和遗传抑制对恩杂鲁胺耐药性前列腺癌细胞的细胞增殖、凋亡和侵袭潜力的影响。通过 DNA 结合、荧光素酶测定和 c-Myc 靶基因的表达来分析 c-Myc 的转录活性。我们发现 5-Lox 调节恩杂鲁胺耐药性前列腺癌细胞中的 c-Myc 信号传导,而 Quiflapon/MK591 或 shRNA 对 5-Lox 的抑制会中断致癌的 c-Myc 信号传导,并通过触发 caspase 介导的细胞凋亡来杀死 ERPC 细胞。有趣的是,在相同的实验条件下,MK591 不会影响正常的非癌细胞。 我们的研究结果表明,抑制 5-Lox 可能成为有效杀死 ERPC 细胞而不伤害正常细胞的有前途的新方法,并表明通过用合适的 5-Lox 杀死和消除 ERPC 细胞,可能可以开发出前列腺癌的长期治疗疗法。 -Lox-抑制剂。
更新日期:2020-04-24
中文翻译:

5-脂氧合酶的药理学和遗传靶向可中断 c-Myc 致癌信号传导,并通过细胞凋亡杀死恩杂鲁胺耐药性前列腺癌细胞。
前列腺癌引起的大部分发病率和死亡率都是由去势抵抗性前列腺癌(CRPC)引起的,这种癌总是在抗雄激素治疗后发生。 FDA 批准的恩杂鲁胺通常用于治疗 CRPC,其通过阻断雄激素受体功能发挥作用。然而,即使在最初的良好反应后,恩杂鲁胺耐药性前列腺癌(ERPC)也会发展,最终导致广泛转移。 ERPC的管理极其困难,因为现有的治疗方案无法有效杀死和消除ERPC细胞。尽管恩杂鲁胺耐药性背后的机制尚未得到正确理解,但已发现 c-Myc 过度激活是一种常见事件,在 ERPC 表型的维持和进展中发挥着重要作用。然而,c-Myc 的直接靶向带来了特殊的问题,因为它的非酶性质,并且非癌细胞也需要一定量的 c-Myc 活性。因此,c-Myc 已成为一个难以捉摸的靶点,需要通过新的药物和策略以癌症特异性的方式进行管理。我们研究了 5-脂氧合酶 (5-Lox) 的药理和遗传抑制对恩杂鲁胺耐药性前列腺癌细胞的细胞增殖、凋亡和侵袭潜力的影响。通过 DNA 结合、荧光素酶测定和 c-Myc 靶基因的表达来分析 c-Myc 的转录活性。我们发现 5-Lox 调节恩杂鲁胺耐药性前列腺癌细胞中的 c-Myc 信号传导,而 Quiflapon/MK591 或 shRNA 对 5-Lox 的抑制会中断致癌的 c-Myc 信号传导,并通过触发 caspase 介导的细胞凋亡来杀死 ERPC 细胞。有趣的是,在相同的实验条件下,MK591 不会影响正常的非癌细胞。 我们的研究结果表明,抑制 5-Lox 可能成为有效杀死 ERPC 细胞而不伤害正常细胞的有前途的新方法,并表明通过用合适的 5-Lox 杀死和消除 ERPC 细胞,可能可以开发出前列腺癌的长期治疗疗法。 -Lox-抑制剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号