当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strong Interface Enhanced Hydrogen Evolution over Molybdenum-Based Catalysts
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-04-20 00:00:00 , DOI: 10.1021/acsaem.0c00045 Zheng Peng 1, 2 , Kaili Wang 1 , Wei Xu 2, 3 , Beibei Wang 1 , Baohua Mao 2 , Yong Han 1 , Chia-Kuang Tsung 4 , Bo Yang 1 , Zhi Liu 1, 2 , Yimin Li 1, 2
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-04-20 00:00:00 , DOI: 10.1021/acsaem.0c00045 Zheng Peng 1, 2 , Kaili Wang 1 , Wei Xu 2, 3 , Beibei Wang 1 , Baohua Mao 2 , Yong Han 1 , Chia-Kuang Tsung 4 , Bo Yang 1 , Zhi Liu 1, 2 , Yimin Li 1, 2
Affiliation
|
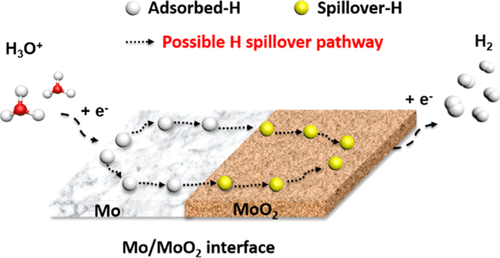
By tuning interfacial structures, we have achieved an extremely small Tafel slope of 34.4 mV dec–1 for the hydrogen evolution reaction (HER) over a molybdenum oxide catalyst in an acidic electrolyte. Such a small Tafel slope indicates the presence of active sites following the Volmer–Tafel mechanism, which is almost exclusively observed on platinum group metals. We attribute this excellent kinetic property to the enhancement effect from the metal/metal oxide (Mo/MoOx) interface in the catalysts. This Mo/MoOx interface was obtained by tuning the hydrogen annealing method. Density functional theory calculations suggest that the hydrogen spillover from the Mo surface to the MoOx surface through an optimized interface will increase the hydrogen coverage on the MoOx surface. Thus, the hydrogen adsorption energy on MoOx can be reduced, making the recombination of the surface hydrogen feasible. Hydrogen temperature-programmed reduction provides clear evidence of hydrogen spillover from Mo to MoO2 at the Mo/MoO2 interfaces. Hence, the above Mo/MoOx interface will also lead to a high HER activity, as demonstrated by the high turnover frequency per active site (at 100, 150, and 200 mV vs the reversible hydrogen electrode, the values are approximately 0.004, 0.249, and 1.398 H2 s–1, respectively). Our study demonstrates a new route to design low-cost-efficient HER catalysts of nonprecious metals by tuning transition metal/oxide interfaces.
中文翻译:

钼基催化剂在强界面上增强了氢的释放
通过调整界面结构,我们在酸性电解质中的氧化钼催化剂上的析氢反应(HER)实现了34.4 mV dec -1的非常小的塔菲尔斜率。如此小的Tafel斜率表明存在遵循Volmer-Tafel机理的活性位点,这几乎只能在铂族金属上观察到。我们将此优异的动力学性能归因于催化剂中金属/金属氧化物(Mo / MoO x)界面的增强作用。通过调整氢退火方法获得该Mo / MoO x界面。密度泛函理论计算表明,氢从Mo表面溢出到MoO x表面通过优化的界面将增加MoO x表面的氢覆盖率。因此,可以减少在MoO x上的氢吸附能,从而使表面氢的再结合成为可能。氢程序升温还原提供了明显的证据,证明了Mo / MoO 2界面上的氢从Mo溢出到MoO 2。因此,上述Mo / MoO x界面也将导致较高的HER活性,如每个活性位点的高转换频率(相对于可逆氢电极,在100、150和200 mV时)所示,其值约为0.004、0.249和1.398 H 2 s –1, 分别)。我们的研究表明,通过调节过渡金属/氧化物界面,可以设计出低成本,低成本的非贵金属HER催化剂的新途径。
更新日期:2020-04-20
中文翻译:

钼基催化剂在强界面上增强了氢的释放
通过调整界面结构,我们在酸性电解质中的氧化钼催化剂上的析氢反应(HER)实现了34.4 mV dec -1的非常小的塔菲尔斜率。如此小的Tafel斜率表明存在遵循Volmer-Tafel机理的活性位点,这几乎只能在铂族金属上观察到。我们将此优异的动力学性能归因于催化剂中金属/金属氧化物(Mo / MoO x)界面的增强作用。通过调整氢退火方法获得该Mo / MoO x界面。密度泛函理论计算表明,氢从Mo表面溢出到MoO x表面通过优化的界面将增加MoO x表面的氢覆盖率。因此,可以减少在MoO x上的氢吸附能,从而使表面氢的再结合成为可能。氢程序升温还原提供了明显的证据,证明了Mo / MoO 2界面上的氢从Mo溢出到MoO 2。因此,上述Mo / MoO x界面也将导致较高的HER活性,如每个活性位点的高转换频率(相对于可逆氢电极,在100、150和200 mV时)所示,其值约为0.004、0.249和1.398 H 2 s –1, 分别)。我们的研究表明,通过调节过渡金属/氧化物界面,可以设计出低成本,低成本的非贵金属HER催化剂的新途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号