当前位置:
X-MOL 学术
›
J. Mater. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triazole functionalized 5,9-dioxa-13b-boranaphtho[3,2,1-de]anthracene: a new family of multi-stimuli responsive materials
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2020-04-18 , DOI: 10.1039/d0tc00865f Guoyun Meng 1, 2, 3, 4, 5 , Tai Peng 6, 7, 8, 9 , Yonggang Shi 1, 2, 3, 4, 5 , Haijun Li 10, 11, 12 , Xiang Wang 10, 11, 12 , Xiaodong Yin 1, 2, 3, 4, 5 , Deng-Tao Yang 10, 11, 12 , Suning Wang 1, 2, 3, 4, 5 , Nan Wang 1, 2, 3, 4, 5
Journal of Materials Chemistry C ( IF 5.7 ) Pub Date : 2020-04-18 , DOI: 10.1039/d0tc00865f Guoyun Meng 1, 2, 3, 4, 5 , Tai Peng 6, 7, 8, 9 , Yonggang Shi 1, 2, 3, 4, 5 , Haijun Li 10, 11, 12 , Xiang Wang 10, 11, 12 , Xiaodong Yin 1, 2, 3, 4, 5 , Deng-Tao Yang 10, 11, 12 , Suning Wang 1, 2, 3, 4, 5 , Nan Wang 1, 2, 3, 4, 5
Affiliation
|
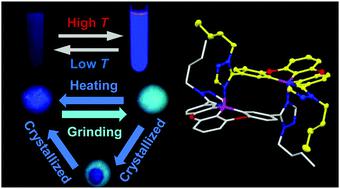
A series of N-heterocycle-tethered organoboranes B1–B5 were synthesized and fully characterized. All these compounds contain a robust 5,9-dioxa-13b-boranaphtho[3,2,1-de]anthracene (DBNA) core which plays a role as a Lewis acid site. Study of the different N-heterocycles as Lewis base moieties reveals that n-butyl-1H-1,2,4-triazolyl functionalized compounds (B1–B3) exhibit the dynamic switching of the intermolecular B–N bond in response to heat stimulus, leading to unusual reversible emission intensity enhancement at elevated temperature, while n-hexyl-1H-1,2,3-triazolyl attached compounds (B4–B5) only display thermal emission quenching behaviors. The different stimuli-responsive properties in solutions may be attributed to the position of the substituent alkyl chains which influence the torsion of the molecules, leading to different affinity of intermolecular B–N interactions. Single crystal X-ray diffraction analysis further confirms the dimeric species formation of B1–B3 in solid state. Furthermore, all compounds exhibit good mechanochromism.
中文翻译:

三唑功能化的5,9-dioxa-13b-硼萘并[3,2,1-de]蒽:新型的多刺激响应材料
合成并充分表征了一系列N杂环连接的有机硼烷B1-B5。所有这些化合物都包含一个坚固的5,9- dioxa -13 b-硼萘并[3,2,1- de ]蒽(DBNA)核,该核起路易斯酸位点的作用。对不同的N-杂环作为路易斯基团的研究表明,正丁基-1 H -1,2,4-三唑基官能化的化合物(B1-B3)表现出分子间B-N键对热刺激的动态转换,导致高温下异常可逆的发射强度增强,而正己基-1 H -1,2,3-三唑基附着的化合物(B4–B5)仅显示热发射猝灭行为。溶液中不同的刺激响应特性可能归因于取代基烷基链的位置,该位置影响分子的扭转,从而导致分子间B–N相互作用的亲和力不同。单晶X射线衍射分析进一步证实了固态B1-B3的二聚体形成。此外,所有化合物均表现出良好的机械致变色作用。
更新日期:2020-06-18
中文翻译:

三唑功能化的5,9-dioxa-13b-硼萘并[3,2,1-de]蒽:新型的多刺激响应材料
合成并充分表征了一系列N杂环连接的有机硼烷B1-B5。所有这些化合物都包含一个坚固的5,9- dioxa -13 b-硼萘并[3,2,1- de ]蒽(DBNA)核,该核起路易斯酸位点的作用。对不同的N-杂环作为路易斯基团的研究表明,正丁基-1 H -1,2,4-三唑基官能化的化合物(B1-B3)表现出分子间B-N键对热刺激的动态转换,导致高温下异常可逆的发射强度增强,而正己基-1 H -1,2,3-三唑基附着的化合物(B4–B5)仅显示热发射猝灭行为。溶液中不同的刺激响应特性可能归因于取代基烷基链的位置,该位置影响分子的扭转,从而导致分子间B–N相互作用的亲和力不同。单晶X射线衍射分析进一步证实了固态B1-B3的二聚体形成。此外,所有化合物均表现出良好的机械致变色作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号