当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of diaminopyrimidine-carboxamide derivatives as JAK3 inhibitors.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-04-18 , DOI: 10.1016/j.bioorg.2020.103851 Rajesh Bahekar 1 , Nandini Panchal 2 , Shubhangi Soman 3 , Jigar Desai 1 , Dipam Patel 1 , Anil Argade 1 , Archana Gite 1 , Sanjay Gite 1 , Bhaumin Patel 1 , Jeevan Kumar 4 , Sachchidanand S 4 , Harilal Patel 5 , Rajesh Sundar 5 , Abhijit Chatterjee 5 , Jogeswar Mahapatra 5 , Hoshang Patel 6 , Krishnarup Ghoshdastidar 6 , Debdutta Bandyopadhyay 6 , Ranjit C Desai 1
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-04-18 , DOI: 10.1016/j.bioorg.2020.103851 Rajesh Bahekar 1 , Nandini Panchal 2 , Shubhangi Soman 3 , Jigar Desai 1 , Dipam Patel 1 , Anil Argade 1 , Archana Gite 1 , Sanjay Gite 1 , Bhaumin Patel 1 , Jeevan Kumar 4 , Sachchidanand S 4 , Harilal Patel 5 , Rajesh Sundar 5 , Abhijit Chatterjee 5 , Jogeswar Mahapatra 5 , Hoshang Patel 6 , Krishnarup Ghoshdastidar 6 , Debdutta Bandyopadhyay 6 , Ranjit C Desai 1
Affiliation
|
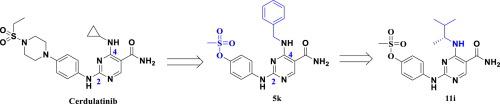
Selective inhibition of janus kinase (JAK) has been identified as an important strategy for the treatment of autoimmune disorders. Optimization at the C2 and C4-positions of pyrimidine ring of Cerdulatinib led to the discovery of a potent and orally bioavailable 2,4-diaminopyrimidine-5-carboxamide based JAK3 selective inhibitor (11i). A cellular selectivity study further confirmed that 11i preferentially inhibits JAK3 over JAK1, in JAK/STAT signaling pathway. Compound 11i showed good anti-arthritic activity, which could be correlated with its improved oral bioavailability. In the repeat dose acute toxicity study, 11i showed no adverse changes related to gross pathology and clinical signs, indicating that the new class JAK3 selective inhibitor could be viable therapeutic option for the treatment of rheumatoid arthritis.
中文翻译:

发现作为JAK3抑制剂的二氨基嘧啶-羧酰胺衍生物。
选择性抑制janus激酶(JAK)已被确定为治疗自身免疫性疾病的重要策略。在Cerdulatinib的嘧啶环的C2和C4位置上的优化导致发现了一种有效的,口服可生物利用的基于2,4-二氨基嘧啶-5-羧酰胺的JAK3选择性抑制剂(11i)。细胞选择性研究进一步证实,在JAK / STAT信号通路中,11i比JAK1优先抑制JAK3。化合物11i显示出良好的抗关节炎活性,这与其改善的口服生物利用度有关。在重复剂量急性毒性研究中,11i没有显示出与总体病理学和临床体征有关的不利变化,表明新的JAK3类选择性抑制剂可能是治疗类风湿关节炎的可行治疗选择。
更新日期:2020-04-20
中文翻译:

发现作为JAK3抑制剂的二氨基嘧啶-羧酰胺衍生物。
选择性抑制janus激酶(JAK)已被确定为治疗自身免疫性疾病的重要策略。在Cerdulatinib的嘧啶环的C2和C4位置上的优化导致发现了一种有效的,口服可生物利用的基于2,4-二氨基嘧啶-5-羧酰胺的JAK3选择性抑制剂(11i)。细胞选择性研究进一步证实,在JAK / STAT信号通路中,11i比JAK1优先抑制JAK3。化合物11i显示出良好的抗关节炎活性,这与其改善的口服生物利用度有关。在重复剂量急性毒性研究中,11i没有显示出与总体病理学和临床体征有关的不利变化,表明新的JAK3类选择性抑制剂可能是治疗类风湿关节炎的可行治疗选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号