Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-04-19 , DOI: 10.1016/j.jmb.2020.04.006 Laine Lysyk 1 , Raelynn Brassard 1 , Nicolas Touret 1 , M Joanne Lemieux 1
|
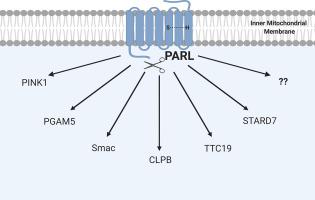
Intramembrane proteolysis, although once a controversial concept, is a widely studied field. Four classes of intramembrane proteases have been identified and are classified by their catalytic mechanism of peptide bond hydrolysis: metallo, glutamyl, aspartyl, and serine proteases. One of the most studied of these classes is the rhomboid superfamily of serine intramembrane proteases. Rhomboids consist of six or seven transmembrane segments that form a helical bundle within the membrane and are involved in a multitude of cellular processes. These proteases are characterized by a catalytic dyad composed of a serine and a histidine residue, which distinguishes them from classical serine proteases wherein a catalytic triad is utilized. Of all currently identified rhomboid proteases, one that is of great interest is the mammalian mitochondrial rhomboid protease PARL. Most well known for its processing of the kinase PINK1 and potential link to Parkinson's disease, PARL has been shown to cleave a variety of substrates within the cell including PGAM5, Smac, TTC19, and others. While recent proteomic studies have provided insight on new potential substrates of PARL, its regulation, activity, and role in maintaining mitochondrial homeostasis remain largely unknown.
中文翻译:

PARL蛋白酶:内线粒体膜的膜内蛋白水解现象。
膜内蛋白水解虽然曾经是一个有争议的概念,但却是一个广泛研究的领域。已鉴定出四类膜内蛋白酶,并通过其肽键水解的催化机理对其进行了分类:金属蛋白酶,谷氨酰基酶,天冬氨酰蛋白酶和丝氨酸蛋白酶。这些类别中研究最多的之一是丝氨酸膜内蛋白酶的菱形超家族。菱形由六个或七个跨膜段组成,它们在膜内形成螺旋束,并参与许多细胞过程。这些蛋白酶的特征在于由丝氨酸和组氨酸残基组成的催化二聚体,这与经典的丝氨酸蛋白酶不同,在传统的丝氨酸蛋白酶中使用了催化三联体。在所有目前确定的菱形蛋白酶中,令人关注的是哺乳动物的线粒体菱形蛋白酶PARL。PARL以其对PINK1激酶的加工以及与帕金森氏病的潜在联系而闻名,已被证明可裂解细胞内的各种底物,包括PGAM5,Smac,TTC19等。虽然最近的蛋白质组学研究提供了关于PARL的新潜在底物的见解,但其调控,活性以及在维持线粒体体内平衡中的作用仍然未知。































 京公网安备 11010802027423号
京公网安备 11010802027423号