Electrochimica Acta ( IF 5.5 ) Pub Date : 2020-04-18 , DOI: 10.1016/j.electacta.2020.136271 Anna T.S. Freiberg , Johannes Sicklinger , Sophie Solchenbach , Hubert A. Gasteiger
|
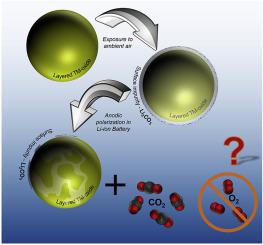
Layered lithium transition metal oxides are state-of-the-art cathode materials for Li-ion batteries. Nickel-rich layered oxides suffer from high surface reactivity toward ambient air. Besides hydroxides, carbonates are known to be the major surface impurities formed. While the decomposition of Li2CO3 in a battery cell has been studied extensively, the mechanistic aspects of its decomposition during cell formation/cycling are still highly controversial.
The decomposition reaction of Li2CO3 in a standard Li-ion battery electrolyte is studied by on-line electrochemical mass spectrometry, employing an electrode only consisting of Li2CO3 and conductive carbon. By modifying the electrode configurations in the cell, we are able to show that the decomposition of Li2CO3 occurs as a chemical process without any direct electrochemical oxidation of the Li2CO3 particles. Their decomposition proceeds by a chemical process via protons that are formed upon anodic oxidation of the electrolyte solvent and of trace impurities in alkyl carbonate based electrolytes. By adding common impurities in Li-ion battery electrolytes as ethanol and ethylene glycol, whose electrochemical oxidation at rather low anodic potentials (≈3.5 V vs Li+/Li) results in the formation of protons, the onset of CO2 evolution from Li2CO3 is accordingly shifted to such low potentials. Tracing the proton-induced LiPF6 decomposition products PF5/POF3, the formation of protons can be followed quantitatively and a direct correlation with the CO2 produced by the proton-induced Li2CO3 decomposition is shown. Implications of these findings for transition metal oxide based cathode materials in Li-ion batteries are discussed based on the here found decomposition mechanism.
中文翻译:

电解质和电解质杂质的电化学氧化导致锂离子电池中的Li 2 CO 3分解
层状锂过渡金属氧化物是用于锂离子电池的最新阴极材料。富镍层状氧化物对环境空气的表面反应性很高。除氢氧化物外,碳酸盐是形成的主要表面杂质。尽管已经广泛研究了Li 2 CO 3在电池单元中的分解,但是在电池形成/循环期间其分解的机理方面仍存在很大争议。
采用仅由Li 2 CO 3和导电碳组成的电极,通过在线电化学质谱法研究了标准锂离子电池电解质中Li 2 CO 3的分解反应。通过修改电池中的电极配置,我们能够证明Li 2 CO 3的分解是化学过程发生的,而Li 2 CO 3颗粒没有任何直接的电化学氧化。它们的分解是通过化学作用进行的通过质子化过程,质子是在对电解质溶剂和烷基碳酸酯类电解质中的痕量杂质进行阳极氧化后形成的。通过在锂离子电池电解质中添加乙醇和乙二醇等常见杂质,它们在相当低的阳极电势下(约3.5 V vs Li + / Li)发生电化学氧化,导致质子的形成,CO 2从Li 2析出。因此,CO 3转变为如此低的电位。跟踪质子诱导的LiPF 6分解产物PF 5 / POF 3,可以定量跟踪质子的形成,并与质子诱导的Li产生的CO 2直接相关。显示了2 CO 3分解。基于此处发现的分解机理,讨论了这些发现对锂离子电池中基于过渡金属氧化物的正极材料的影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号