当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Intrinsic Nature of Persulfate Activation and N-Doping in Carbocatalysis.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-04-29 , DOI: 10.1021/acs.est.0c01161 Wei Ren 1, 2 , Gang Nie 1, 2 , Peng Zhou 2, 3 , Hui Zhang 1 , Xiaoguang Duan 2 , Shaobin Wang 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-04-29 , DOI: 10.1021/acs.est.0c01161 Wei Ren 1, 2 , Gang Nie 1, 2 , Peng Zhou 2, 3 , Hui Zhang 1 , Xiaoguang Duan 2 , Shaobin Wang 2
Affiliation
|
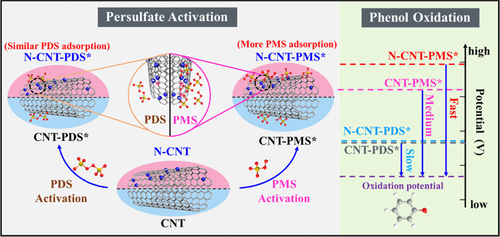
Persulfates activation by carbon nanotubes (CNT) has been evidenced as nonradical systems for oxidation of organic pollutants. Peroxymonosulfate (PMS) and peroxydisulfate (PDS) possess discrepant atomic structures and redox potentials, while the nature of their distinct behaviors in carbocatalytic activation has not been investigated. Herein, we illustrated that the roles of nitrogen species in CNT-based persulfate systems are intrinsically different. In PMS activation mediated by nitrogen-doped CNT (N-CNT), surface chemical modification (N-doping) can profoundly promote the adsorption quantity of PMS, consequently elevate potential of derived nonradical N-CNT-PMS* complexes, and boost organic oxidation efficiency via an electron-transfer regime. In contrast, PDS adsorption was not enhanced upon incorporating N into CNT due to the limited equilibrium adsorption quantity of PDS, leading to a relatively lower oxidative potential of PDS/N-CNT system and a mediocre degradation rate. However, with equivalent persulfate adsorption on N-CNT at a low quantity, PDS/N-CNT exhibited a stronger oxidizing capacity than PMS/N-CNT because of the intrinsic higher redox potential of PDS than PMS. The oxidation rates of the two systems were in great linearity with the potentials of carbon-persulfate* complexes, suggesting N-CNT activation of PMS and PDS shared the similar electron-transfer oxidation mechanism. Therefore, this study provides new insights into the intrinsic roles of heteroatom doping in nanocarbons for persulfates activation and unveils the principles for a rational design of reaction-oriented carbocatalysts for persulfate-based advanced oxidation processes.
中文翻译:

碳催化过程中过硫酸盐活化和氮掺杂的内在本质。
碳纳米管(CNT)的过硫酸盐活化已被证明是氧化有机污染物的非自由基体系。过氧一硫酸盐(PMS)和过氧二硫酸盐(PDS)具有不同的原子结构和氧化还原电势,但尚未研究其在碳催化活化中的独特行为。在这里,我们说明了氮物种在基于CNT的过硫酸盐体系中的作用本质上是不同的。在氮掺杂CNT(N-CNT)介导的PMS活化中,表面化学改性(N-掺杂)可以显着提高PMS的吸附量,从而提高衍生的非自由基N-CNT-PMS *复合物的电势,并促进有机氧化通过电子转移机制的效率。相反,由于有限的PDS平衡吸附量,在将N掺入CNT后,PDS的吸附作用并未增强,导致PDS / N-CNT系统的氧化电位相对较低,降解速度中等。但是,当过硫酸盐少量吸附在N-CNT上时,由于PDS固有的氧化还原电势高于PMS,因此PDS / N-CNT的氧化能力强于PMS / N-CNT。这两个系统的氧化速率与过硫酸碳*络合物的电势呈线性关系,这表明PMS和PDS的N-CNT活化具有相似的电子转移氧化机理。因此,
更新日期:2020-04-17
中文翻译:

碳催化过程中过硫酸盐活化和氮掺杂的内在本质。
碳纳米管(CNT)的过硫酸盐活化已被证明是氧化有机污染物的非自由基体系。过氧一硫酸盐(PMS)和过氧二硫酸盐(PDS)具有不同的原子结构和氧化还原电势,但尚未研究其在碳催化活化中的独特行为。在这里,我们说明了氮物种在基于CNT的过硫酸盐体系中的作用本质上是不同的。在氮掺杂CNT(N-CNT)介导的PMS活化中,表面化学改性(N-掺杂)可以显着提高PMS的吸附量,从而提高衍生的非自由基N-CNT-PMS *复合物的电势,并促进有机氧化通过电子转移机制的效率。相反,由于有限的PDS平衡吸附量,在将N掺入CNT后,PDS的吸附作用并未增强,导致PDS / N-CNT系统的氧化电位相对较低,降解速度中等。但是,当过硫酸盐少量吸附在N-CNT上时,由于PDS固有的氧化还原电势高于PMS,因此PDS / N-CNT的氧化能力强于PMS / N-CNT。这两个系统的氧化速率与过硫酸碳*络合物的电势呈线性关系,这表明PMS和PDS的N-CNT活化具有相似的电子转移氧化机理。因此,































 京公网安备 11010802027423号
京公网安备 11010802027423号