Chem ( IF 19.1 ) Pub Date : 2020-04-17 , DOI: 10.1016/j.chempr.2020.03.021 Min Wu , Yan Jing , Andrew A. Wong , Eric M. Fell , Shijian Jin , Zhijiang Tang , Roy G. Gordon , Michael J. Aziz
|
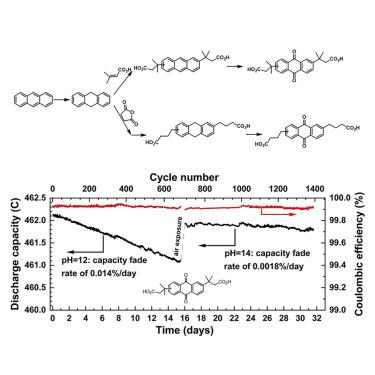
Synthetic cost and long-term stability remain two of the most challenging barriers for the utilization of redox-active organic molecules in redox flow batteries for grid-scale energy storage. Starting from potentially inexpensive 9,10-dihydroanthracene, we developed a new synthetic approach for two extremely stable anthraquinone negolytes, i.e., 3,3′-(9,10-anthraquinone-diyl)bis(3-methylbutanoic acid) (DPivOHAQ) and 4,4′-(9,10-anthraquinone-diyl)dibutanoic acid (DBAQ). Pairing with a ferrocyanide posolyte at pH 12, DPivOHAQ and DBAQ can transfer up to 1.4 and 2 M electrons with capacity fade rates of 0.014% per day and 0.0084% per day, respectively, and exhibit 1.0 V of open-circuit voltage. By adjusting the supporting electrolytes to pH 14, DPivOHAQ exhibited a record low capacity fade rate of <1% per year. We attribute the capacity loss of these flow batteries primarily to the formation of anthrone, which can be suppressed by increasing the pH of the electrolyte and reversed by exposure to air.
中文翻译:

由前驱体合成的极其稳定的蒽醌共沸物
合成成本和长期稳定性仍然是在氧化还原液流电池中用于电网规模储能的氧化还原活性有机分子利用的两个最具挑战性的障碍。从可能廉价的9,10-二氢蒽开始,我们开发了一种新的合成方法,用于两种极其稳定的蒽醌正溶剂,即3,3'-(9,10-蒽醌-二基)双(3-甲基丁酸)(DPivOHAQ)和4,4'-(9,10-蒽醌-二基)二丁酸(DBAQ)。与pH 12的亚铁氰化物多聚体配对,DPivOHAQ和DBAQ可以转移多达1.4和2 M个电子,容量衰减率分别为每天0.014%和0.0084%/天,并显示1.0 V的开路电压。通过将支持电解质的pH值调整为14,DPivOHAQ表现出了创纪录的低容量衰落率,每年<1%。































 京公网安备 11010802027423号
京公网安备 11010802027423号