Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bio-assisted synthesized Pd nanoparticles supported on ionic liquid decorated magnetic halloysite: an efficient catalyst for degradation of dyes.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-04-16 , DOI: 10.1038/s41598-020-63558-8 Samahe Sadjadi 1 , Pourya Mohammadi 2 , Majid Heravi 2
Scientific Reports ( IF 3.8 ) Pub Date : 2020-04-16 , DOI: 10.1038/s41598-020-63558-8 Samahe Sadjadi 1 , Pourya Mohammadi 2 , Majid Heravi 2
Affiliation
|
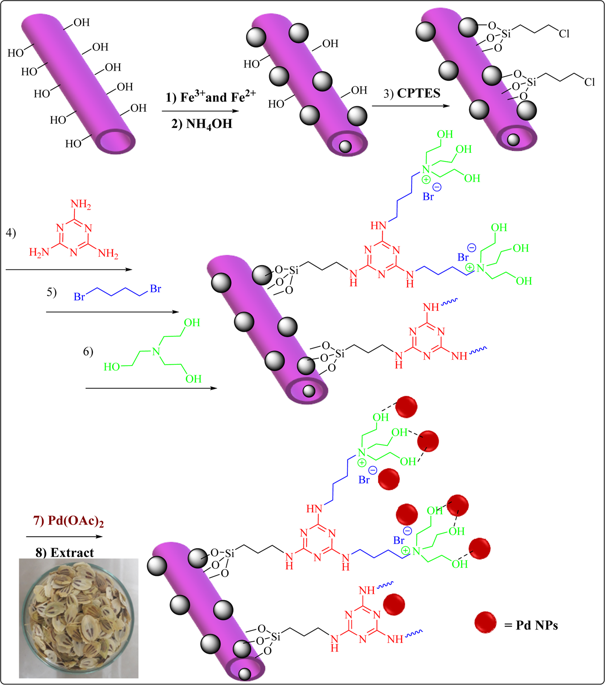
Using natural materials, i.e. halloysite nanoclay that is a biocompatible naturally occurring clay and Heracleum persicum extract that can serve as a green reducing agent, a novel magnetic catalyst, Fe3O4/Hal-Mel-TEA(IL)-Pd, has been designed and fabricated. To prepare the catalyst, halloysite was first magnetized (magnetic particles with mean diameter of 13.06 ± 3.1 nm) and then surface functionalized with melamine, 1,4 dibromobutane and triethanolamine to provide ionic liquid on the halloysite surface (5 wt%). The latter was then used as a support to immobilize Pd nanoparticles that were reduced by Heracleum persicum extract. The characterization of the catalyst established that the loading of Pd in Fe3O4/Hal-Mel-TEA(IL)-Pd was very low (0.93 wt%) and its specific surface area was 63 m2g-1. Moreover, the catalyst showed magnetic property (Ms = 19.75 emu g-1) and could be magnetically separated from the reaction. The catalytic performance of the magnetic catalyst for reductive degradation of methyl orange and rhodamine B in the presence of NaBH4 in aqueous media was investigated. The activation energy, enthalpy, and entropy for the reduction of methyl orange were estimated as 42.02 kJ mol-1, 39.40 kJ mol-1, and -139.06 J mol-1 K-1, respectively. These values for rhodamine B were calculated as 39.97 kJ mol-1, 34.33 kJ mol-1, and -155.18 Jmol-1K-1, respectively. Notably, Fe3O4/Hal-Mel-TEA(IL)-Pd could be reused for eight reaction runs with negligible loss of the catalytic activity (~3%) and Pd leaching (0.01 wt% of the initial loading).
中文翻译:

负载在离子液体修饰的磁性埃洛石上的生物辅助合成的Pd纳米颗粒:一种有效的染料降解催化剂。
使用天然材料,即生物相容的天然粘土埃洛石纳米粘土和可用作绿色还原剂的Peracum提取物,设计并制造了新型磁性催化剂Fe3O4 / Hal-Mel-TEA(IL)-Pd。 。为了制备催化剂,首先将埃洛石磁化(平均直径为13.06±3.1 nm的磁性颗粒),然后用三聚氰胺,1,4二溴丁烷和三乙醇胺对表面进行官能化,以在埃洛石表面上提供离子液体(5 wt%)。然后将后者用作支撑物,以固定被Heracleum persicum提取物还原的Pd纳米颗粒。催化剂的特性表明,Fe3O4 / Hal-Mel-TEA(IL)-Pd中的Pd含量非常低(0.93 wt%),比表面积为63 m2g-1。此外,催化剂显示出磁性(Ms = 19.75 emu g-1),可以从反应中磁性分离。研究了磁性催化剂在NaBH4存在下在水性介质中还原还原甲基橙和若丹明B的催化性能。甲基橙还原的活化能,焓和熵分别估计为42.02 kJ mol-1、39.40 kJ mol-1和-139.06 J mol-1 K-1。罗丹明B的这些值分别计算为39.97 kJ mol-1、34.33 kJ mol-1和-155.18 Jmol-1K-1。值得注意的是,Fe3O4 / Hal-Mel-TEA(IL)-Pd可以重复使用八次反应,而催化活性损失(〜3%)和Pd浸出(初始负载的0.01 wt%)可以忽略不计。研究了磁性催化剂在NaBH4存在下在水性介质中还原还原甲基橙和若丹明B的催化性能。甲基橙还原的活化能,焓和熵分别估计为42.02 kJ mol-1、39.40 kJ mol-1和-139.06 J mol-1 K-1。罗丹明B的这些值分别计算为39.97 kJ mol-1、34.33 kJ mol-1和-155.18 Jmol-1K-1。值得注意的是,Fe3O4 / Hal-Mel-TEA(IL)-Pd可以重复使用八次反应,而催化活性损失(〜3%)和Pd浸出(初始负载的0.01 wt%)可以忽略不计。研究了磁性催化剂在NaBH4存在下在水性介质中还原还原甲基橙和若丹明B的催化性能。甲基橙还原的活化能,焓和熵分别估计为42.02 kJ mol-1、39.40 kJ mol-1和-139.06 J mol-1 K-1。罗丹明B的这些值分别计算为39.97 kJ mol-1、34.33 kJ mol-1和-155.18 Jmol-1K-1。值得注意的是,Fe3O4 / Hal-Mel-TEA(IL)-Pd可以重复使用八次反应,而催化活性损失(〜3%)和Pd浸出(初始负载的0.01 wt%)可以忽略不计。还原甲基橙的熵和熵估计分别为42.02 kJ mol-1、39.40 kJ mol-1和-139.06 J mol-1 K-1。罗丹明B的这些值分别计算为39.97 kJ mol-1、34.33 kJ mol-1和-155.18 Jmol-1K-1。值得注意的是,Fe3O4 / Hal-Mel-TEA(IL)-Pd可以重复使用八次反应,而催化活性损失(〜3%)和Pd浸出(初始负载的0.01 wt%)可以忽略不计。还原甲基橙的熵和熵估计分别为42.02 kJ mol-1、39.40 kJ mol-1和-139.06 J mol-1 K-1。罗丹明B的这些值分别计算为39.97 kJ mol-1、34.33 kJ mol-1和-155.18 Jmol-1K-1。值得注意的是,Fe3O4 / Hal-Mel-TEA(IL)-Pd可以重复使用八次反应,而催化活性损失(〜3%)和Pd浸出(初始负载的0.01 wt%)可以忽略不计。
更新日期:2020-04-24
中文翻译:

负载在离子液体修饰的磁性埃洛石上的生物辅助合成的Pd纳米颗粒:一种有效的染料降解催化剂。
使用天然材料,即生物相容的天然粘土埃洛石纳米粘土和可用作绿色还原剂的Peracum提取物,设计并制造了新型磁性催化剂Fe3O4 / Hal-Mel-TEA(IL)-Pd。 。为了制备催化剂,首先将埃洛石磁化(平均直径为13.06±3.1 nm的磁性颗粒),然后用三聚氰胺,1,4二溴丁烷和三乙醇胺对表面进行官能化,以在埃洛石表面上提供离子液体(5 wt%)。然后将后者用作支撑物,以固定被Heracleum persicum提取物还原的Pd纳米颗粒。催化剂的特性表明,Fe3O4 / Hal-Mel-TEA(IL)-Pd中的Pd含量非常低(0.93 wt%),比表面积为63 m2g-1。此外,催化剂显示出磁性(Ms = 19.75 emu g-1),可以从反应中磁性分离。研究了磁性催化剂在NaBH4存在下在水性介质中还原还原甲基橙和若丹明B的催化性能。甲基橙还原的活化能,焓和熵分别估计为42.02 kJ mol-1、39.40 kJ mol-1和-139.06 J mol-1 K-1。罗丹明B的这些值分别计算为39.97 kJ mol-1、34.33 kJ mol-1和-155.18 Jmol-1K-1。值得注意的是,Fe3O4 / Hal-Mel-TEA(IL)-Pd可以重复使用八次反应,而催化活性损失(〜3%)和Pd浸出(初始负载的0.01 wt%)可以忽略不计。研究了磁性催化剂在NaBH4存在下在水性介质中还原还原甲基橙和若丹明B的催化性能。甲基橙还原的活化能,焓和熵分别估计为42.02 kJ mol-1、39.40 kJ mol-1和-139.06 J mol-1 K-1。罗丹明B的这些值分别计算为39.97 kJ mol-1、34.33 kJ mol-1和-155.18 Jmol-1K-1。值得注意的是,Fe3O4 / Hal-Mel-TEA(IL)-Pd可以重复使用八次反应,而催化活性损失(〜3%)和Pd浸出(初始负载的0.01 wt%)可以忽略不计。研究了磁性催化剂在NaBH4存在下在水性介质中还原还原甲基橙和若丹明B的催化性能。甲基橙还原的活化能,焓和熵分别估计为42.02 kJ mol-1、39.40 kJ mol-1和-139.06 J mol-1 K-1。罗丹明B的这些值分别计算为39.97 kJ mol-1、34.33 kJ mol-1和-155.18 Jmol-1K-1。值得注意的是,Fe3O4 / Hal-Mel-TEA(IL)-Pd可以重复使用八次反应,而催化活性损失(〜3%)和Pd浸出(初始负载的0.01 wt%)可以忽略不计。还原甲基橙的熵和熵估计分别为42.02 kJ mol-1、39.40 kJ mol-1和-139.06 J mol-1 K-1。罗丹明B的这些值分别计算为39.97 kJ mol-1、34.33 kJ mol-1和-155.18 Jmol-1K-1。值得注意的是,Fe3O4 / Hal-Mel-TEA(IL)-Pd可以重复使用八次反应,而催化活性损失(〜3%)和Pd浸出(初始负载的0.01 wt%)可以忽略不计。还原甲基橙的熵和熵估计分别为42.02 kJ mol-1、39.40 kJ mol-1和-139.06 J mol-1 K-1。罗丹明B的这些值分别计算为39.97 kJ mol-1、34.33 kJ mol-1和-155.18 Jmol-1K-1。值得注意的是,Fe3O4 / Hal-Mel-TEA(IL)-Pd可以重复使用八次反应,而催化活性损失(〜3%)和Pd浸出(初始负载的0.01 wt%)可以忽略不计。































 京公网安备 11010802027423号
京公网安备 11010802027423号