Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization.
Cell ( IF 45.5 ) Pub Date : 2020-04-16 , DOI: 10.1016/j.cell.2020.03.050 David W Sanders 1 , Nancy Kedersha 2 , Daniel S W Lee 1 , Amy R Strom 1 , Victoria Drake 1 , Joshua A Riback 1 , Dan Bracha 1 , Jorine M Eeftens 1 , Allana Iwanicki 1 , Alicia Wang 1 , Ming-Tzo Wei 1 , Gena Whitney 1 , Shawn M Lyons 3 , Paul Anderson 2 , William M Jacobs 4 , Pavel Ivanov 2 , Clifford P Brangwynne 5
Cell ( IF 45.5 ) Pub Date : 2020-04-16 , DOI: 10.1016/j.cell.2020.03.050 David W Sanders 1 , Nancy Kedersha 2 , Daniel S W Lee 1 , Amy R Strom 1 , Victoria Drake 1 , Joshua A Riback 1 , Dan Bracha 1 , Jorine M Eeftens 1 , Allana Iwanicki 1 , Alicia Wang 1 , Ming-Tzo Wei 1 , Gena Whitney 1 , Shawn M Lyons 3 , Paul Anderson 2 , William M Jacobs 4 , Pavel Ivanov 2 , Clifford P Brangwynne 5
Affiliation
|
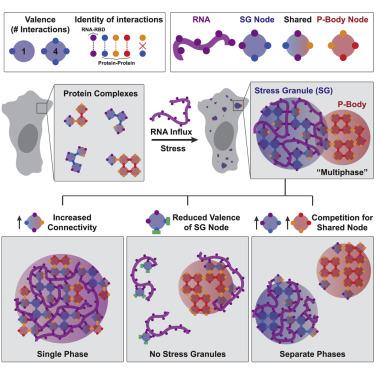
Liquid-liquid phase separation (LLPS) mediates formation of membraneless condensates such as those associated with RNA processing, but the rules that dictate their assembly, substructure, and coexistence with other liquid-like compartments remain elusive. Here, we address the biophysical mechanism of this multiphase organization using quantitative reconstitution of cytoplasmic stress granules (SGs) with attached P-bodies in human cells. Protein-interaction networks can be viewed as interconnected complexes (nodes) of RNA-binding domains (RBDs), whose integrated RNA-binding capacity determines whether LLPS occurs upon RNA influx. Surprisingly, both RBD-RNA specificity and disordered segments of key proteins are non-essential, but modulate multiphase condensation. Instead, stoichiometry-dependent competition between protein networks for connecting nodes determines SG and P-body composition and miscibility, while competitive binding of unconnected proteins disengages networks and prevents LLPS. Inspired by patchy colloid theory, we propose a general framework by which competing networks give rise to compositionally specific and tunable condensates, while relative linkage between nodes underlies multiphase organization.
中文翻译:

竞争性蛋白质-RNA 相互作用网络控制多相细胞内组织。
液-液相分离 (LLPS) 介导无膜冷凝物的形成,例如与 RNA 加工相关的冷凝物,但决定其组装、子结构以及与其他液体样区室共存的规则仍然难以捉摸。在这里,我们利用人体细胞中附着的 P 体的细胞质应激颗粒 (SG) 的定量重建来解决这种多相组织的生物物理机制。蛋白质相互作用网络可以被视为RNA结合域(RBD)的互连复合物(节点),其整合的RNA结合能力决定了RNA流入时是否发生LLPS。令人惊讶的是,RBD-RNA 特异性和关键蛋白的无序片段都不是必需的,但可以调节多相凝结。相反,连接节点的蛋白质网络之间化学计量依赖的竞争决定了 SG 和 P 体的组成和混溶性,而未连接蛋白质的竞争性结合会脱离网络并防止 LLPS。受斑块胶体理论的启发,我们提出了一个通用框架,通过该框架,竞争网络产生成分特定且可调的凝聚体,而节点之间的相对联系是多相组织的基础。
更新日期:2020-04-20
中文翻译:

竞争性蛋白质-RNA 相互作用网络控制多相细胞内组织。
液-液相分离 (LLPS) 介导无膜冷凝物的形成,例如与 RNA 加工相关的冷凝物,但决定其组装、子结构以及与其他液体样区室共存的规则仍然难以捉摸。在这里,我们利用人体细胞中附着的 P 体的细胞质应激颗粒 (SG) 的定量重建来解决这种多相组织的生物物理机制。蛋白质相互作用网络可以被视为RNA结合域(RBD)的互连复合物(节点),其整合的RNA结合能力决定了RNA流入时是否发生LLPS。令人惊讶的是,RBD-RNA 特异性和关键蛋白的无序片段都不是必需的,但可以调节多相凝结。相反,连接节点的蛋白质网络之间化学计量依赖的竞争决定了 SG 和 P 体的组成和混溶性,而未连接蛋白质的竞争性结合会脱离网络并防止 LLPS。受斑块胶体理论的启发,我们提出了一个通用框架,通过该框架,竞争网络产生成分特定且可调的凝聚体,而节点之间的相对联系是多相组织的基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号