当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Manipulating dehydrogenation kinetics through dual-doping Co3N electrode enables highly efficient hydrazine oxidation assisting self-powered H2 production.
Nature Communications ( IF 14.7 ) Pub Date : 2020-04-15 , DOI: 10.1038/s41467-020-15563-8 Yi Liu 1 , Jihua Zhang 2 , Yapeng Li 1 , Qizhu Qian 1 , Ziyun Li 1 , Yin Zhu 1 , Genqiang Zhang 1
Nature Communications ( IF 14.7 ) Pub Date : 2020-04-15 , DOI: 10.1038/s41467-020-15563-8 Yi Liu 1 , Jihua Zhang 2 , Yapeng Li 1 , Qizhu Qian 1 , Ziyun Li 1 , Yin Zhu 1 , Genqiang Zhang 1
Affiliation
|
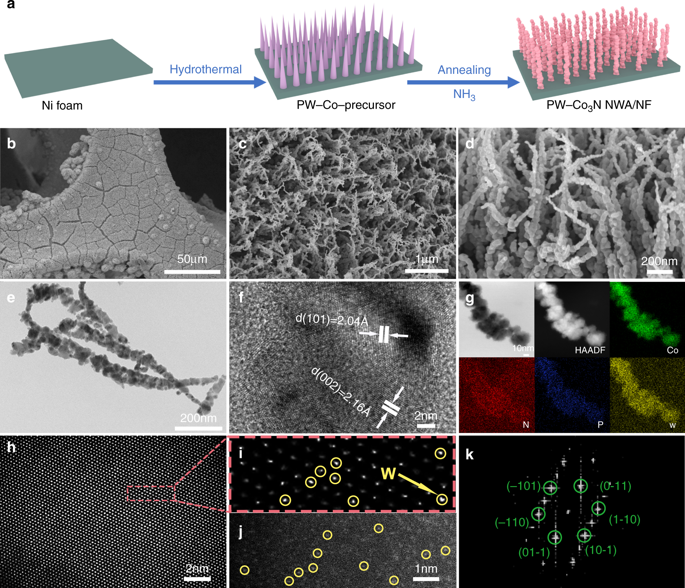
Replacing sluggish oxygen evolution reaction (OER) with hydrazine oxidation reaction (HzOR) to produce hydrogen has been considered as a more energy-efficient strategy than water splitting. However, the relatively high cell voltage in two-electrode system and the required external electric power hinder its scalable applications, especially in mobile devices. Herein, we report a bifunctional P, W co-doped Co3N nanowire array electrode with remarkable catalytic activity towards both HzOR (-55 mV at 10 mA cm-2) and hydrogen evolution reaction (HER, -41 mV at 10 mA cm-2). Inspiringly, a record low cell voltage of 28 mV is required to achieve 10 mA cm-2 in two-electrode system. DFT calculations decipher that the doping optimized H* adsorption/desorption and dehydrogenation kinetics could be the underlying mechanism. Importantly, a self-powered H2 production system by integrating a direct hydrazine fuel cell with a hydrazine splitting electrolyzer can achieve a decent rate of 1.25 mmol h-1 at room temperature.
中文翻译:

通过双掺杂 Co3N 电极控制脱氢动力学,可实现高效肼氧化,协助自供电氢气生产。
用肼氧化反应(HzOR)代替缓慢的析氧反应(OER)来产生氢气被认为是比水分解更节能的策略。然而,两电极系统中相对较高的电池电压和所需的外部电力阻碍了其可扩展应用,特别是在移动设备中。在此,我们报道了一种双功能P、W共掺杂Co3N纳米线阵列电极,对HzOR(10 mA cm-2时-55 mV)和析氢反应(HER,10 mA cm-2时-41 mV)具有显着的催化活性)。令人鼓舞的是,在双电极系统中实现 10 mA cm-2 需要 28 mV 的创纪录低电池电压。 DFT 计算表明,掺杂优化的 H* 吸附/解吸和脱氢动力学可能是潜在的机制。重要的是,通过将直接肼燃料电池与肼裂解电解槽集成的自供电制氢系统可以在室温下实现 1.25 mmol h-1 的不错的产率。
更新日期:2020-04-24
中文翻译:

通过双掺杂 Co3N 电极控制脱氢动力学,可实现高效肼氧化,协助自供电氢气生产。
用肼氧化反应(HzOR)代替缓慢的析氧反应(OER)来产生氢气被认为是比水分解更节能的策略。然而,两电极系统中相对较高的电池电压和所需的外部电力阻碍了其可扩展应用,特别是在移动设备中。在此,我们报道了一种双功能P、W共掺杂Co3N纳米线阵列电极,对HzOR(10 mA cm-2时-55 mV)和析氢反应(HER,10 mA cm-2时-41 mV)具有显着的催化活性)。令人鼓舞的是,在双电极系统中实现 10 mA cm-2 需要 28 mV 的创纪录低电池电压。 DFT 计算表明,掺杂优化的 H* 吸附/解吸和脱氢动力学可能是潜在的机制。重要的是,通过将直接肼燃料电池与肼裂解电解槽集成的自供电制氢系统可以在室温下实现 1.25 mmol h-1 的不错的产率。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号